Fungal growth
1/53
Earn XP
Description and Tags
Lecture 2
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
54 Terms
How many Fungal species
5 million species (although probably more)
> 10 million
Fungal role
Crucial role in environmental mineral and nutrient recycling
ideal model orgnaisms for research
food
beverage
pharmaceutical and biotechnology
Devastate crops
phytopathogens
Cause human disease
mycoses
What is a true fungus?
Should be closer realted to us than to brown seaweed etc
Fungal like:
oomycetes e.g Phytophthora infestans- potato late blight
Plasmodiophorids
similar patterns of growth and nutrition to fungi but less related to us
True fungus:
Penicillium notatum
distantly related to the p.infestans
What are fungi? (structurally)
Eukaryote (can be multi-nucleate)
cell wall: chitin and glucan
multi or unicellular (sometimes)
Vacuoles
like plant vaculose: hydrolytic enzymes, storage
Chloroplast-free zone
most important determining factor
heterotrophs
Roles of Fungi
recycle N and C
cellulose and lignin degradation
hard to digest
saprophytes
assist or destroy crops
arbuscles good
Haustoria bad/pathogenic
save or kill humans
antibiotics
mycosis from bad fungus- aspergillus
can be utilised
Yeast for research
1% are unicellular
Spheroids
small SA per unit volume
wall manufacture is economical
Habitat
plant/animal surfaces
plant exudates
animal mucous membranes
Two main types
budding yeast (Saccharomyces cerevisiae)
fission yeast (Schizosaccharomyces pombe)
Multicellular fungi (filamentous)
‘tube in search of food’- Hyphae
vegetative growth form
emerges from a spore
Habitat
everywhere
What are spores
dispersive propagule (ananolgous to a plant seed)
carries genetic code
Features of spores
Made in vast quantities from differentiated hyphae
Asexual or sexual
Low metabolic activity
Germination trigger
environmental triggers
e.g temperature
Response= imbibing water→ germinating
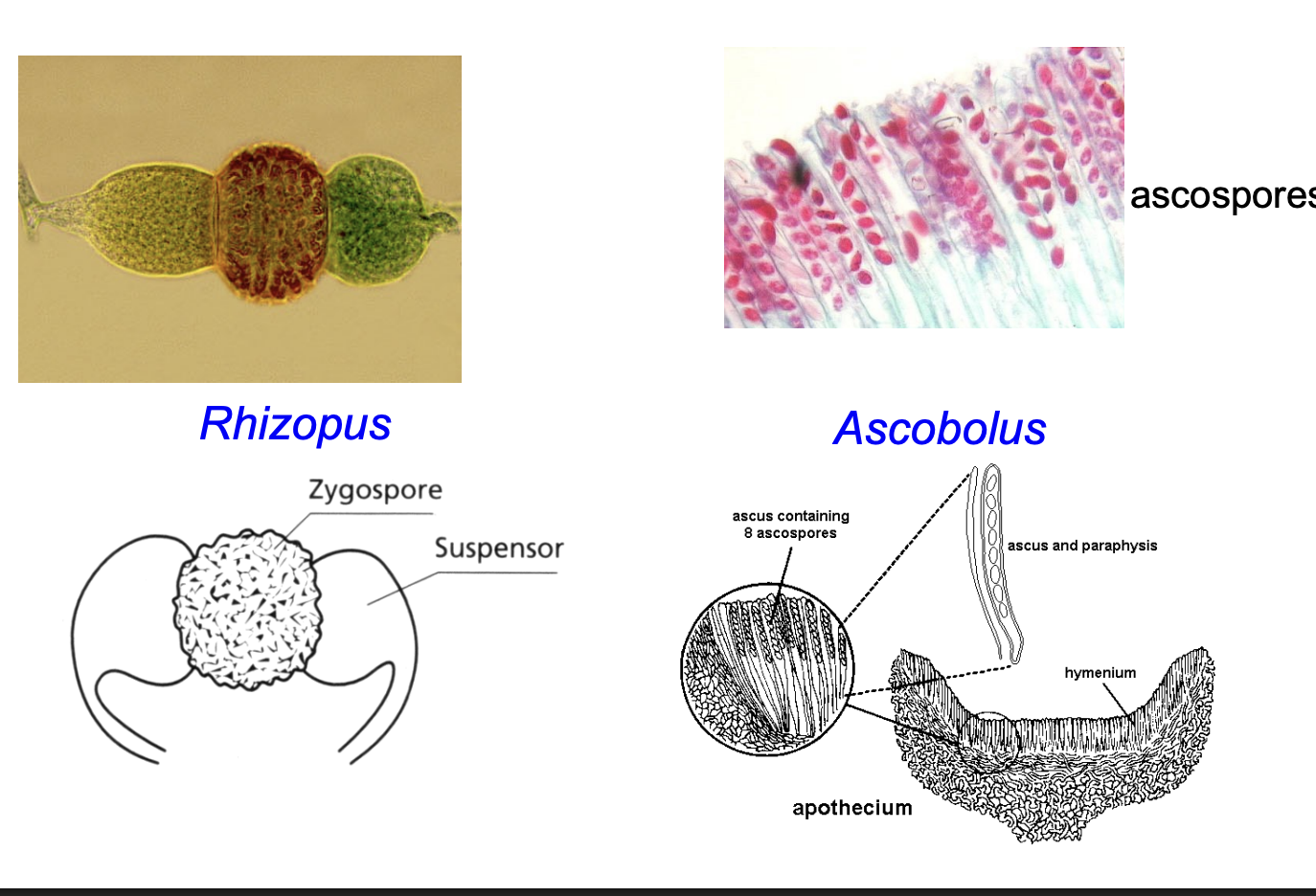
Asexual spores
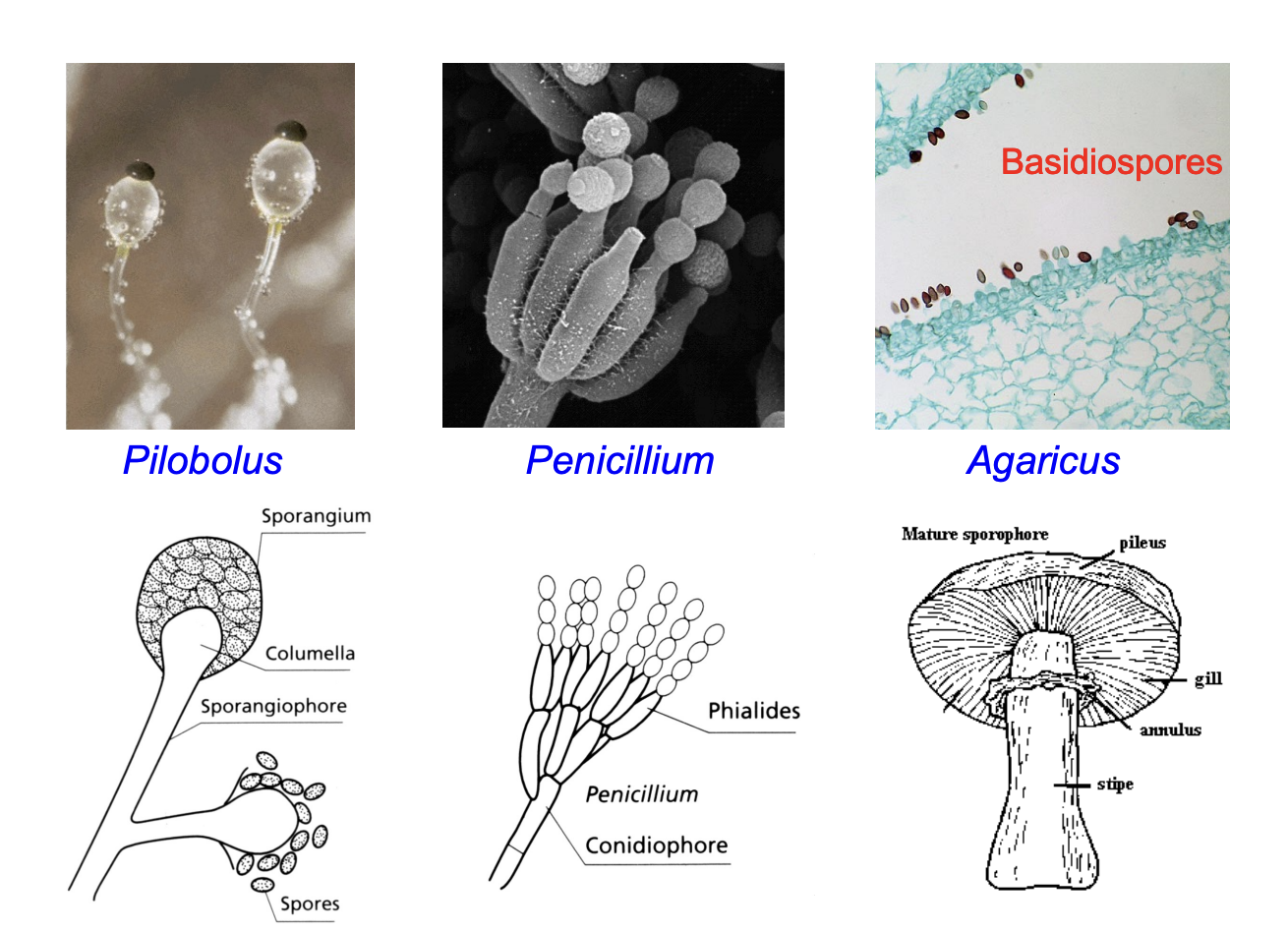
Sexual Spores
What happens during germination of spores
Sphere→ Hypha
to anisotropic (polar) growth
why?
germ tube can locate nutrients
then first cross wall formed
race against time
must locate new food supply
before endogenous reserve are exhausted
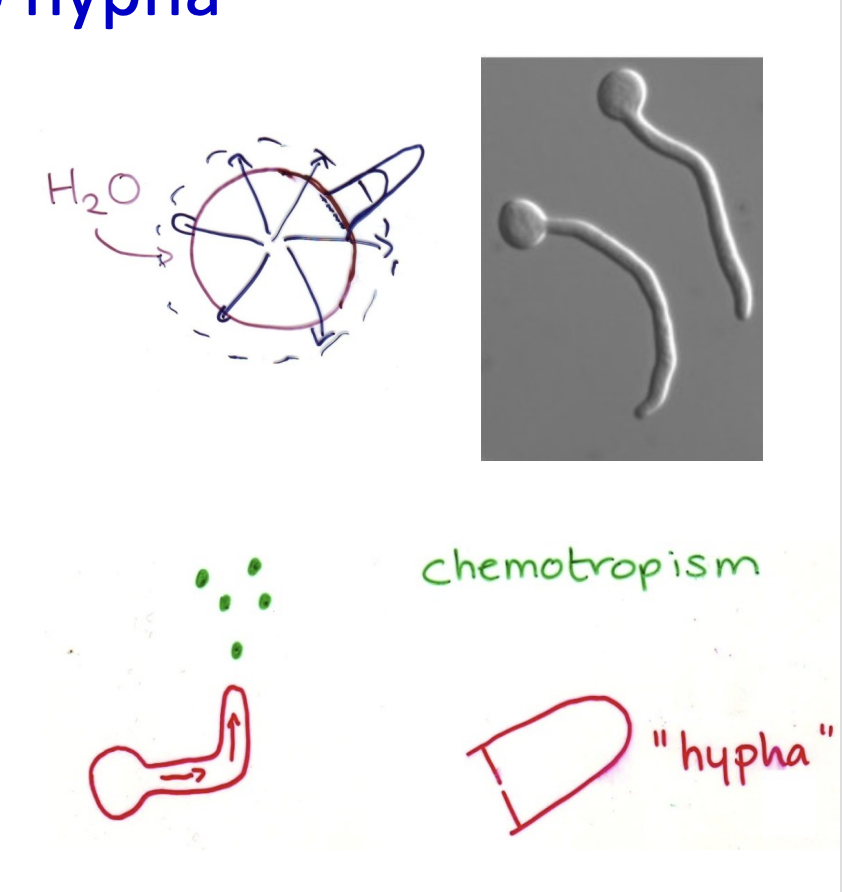
How do the new hyphae find food?
Tropisms
e.g towards amino acids
enable targeted growth towards food supply
found = germ tube matures→ hypha
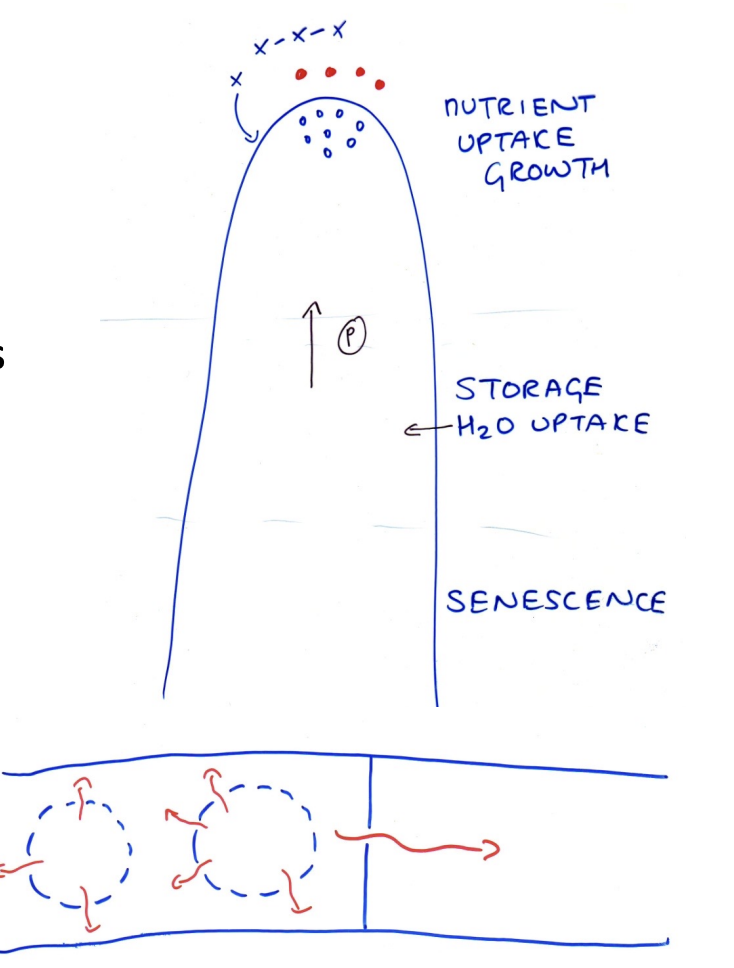
Structure of hypha
Series of zones of specific…
structure and role
Divided into
discrete, regular compartments by cross walls
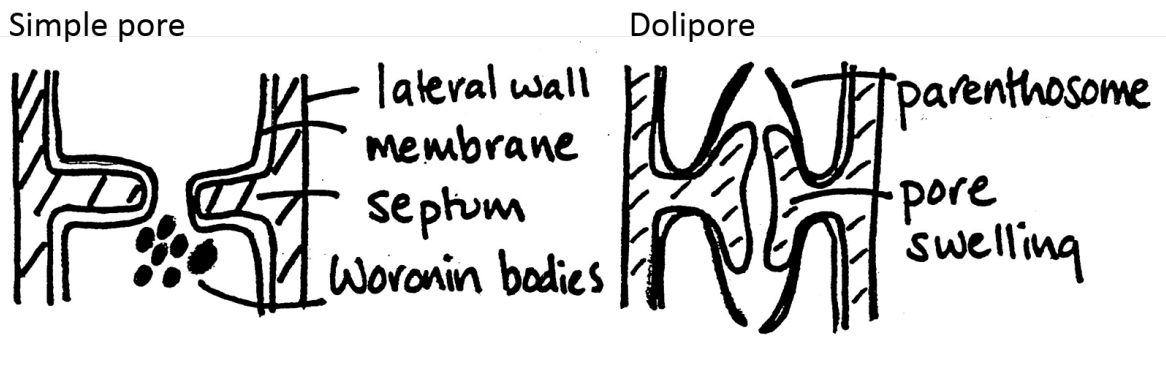
How have protoplasmic continuity?
Pores (septa)
Types:
Woronin bodies: in simple pores
Block the pores
Porteinaceous
Parenthosome: in Dolipores
Apical growth zone: where grow from
restricted to the apex
addition of new materials helps it grow
fungal polar growth
How found out the model of the fungal polar growth
pharaseutical and genetic
manipulation of putative compondents
Generalised model for polar growth: Step 1
Wall component and enzymes to catalyse wall formation
delivered continuously to the tip
via Vesciles
e.g Neurospora crass: 38,000 delivered per minute
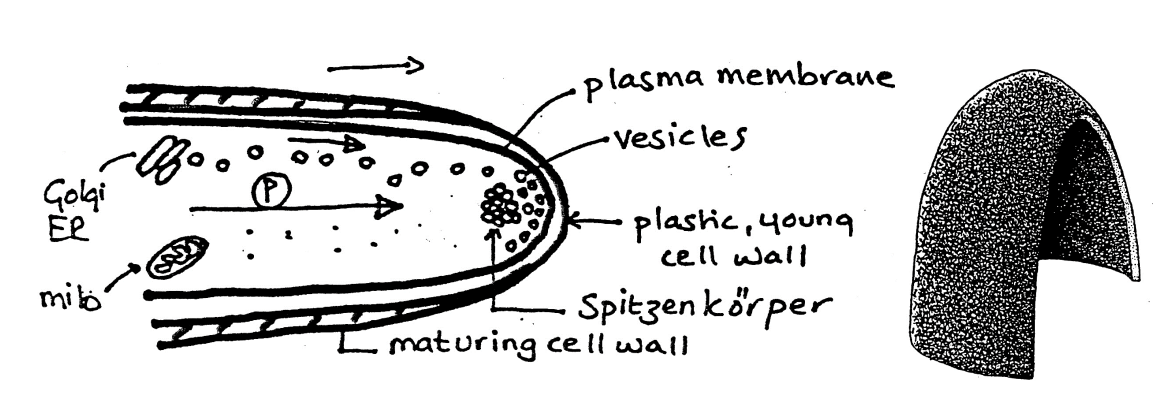
Generalised model for polar growth: Step 2
Vesicles are formed by
sub-apical ER/Goli
directed to the apex by
actin cytoskeleton
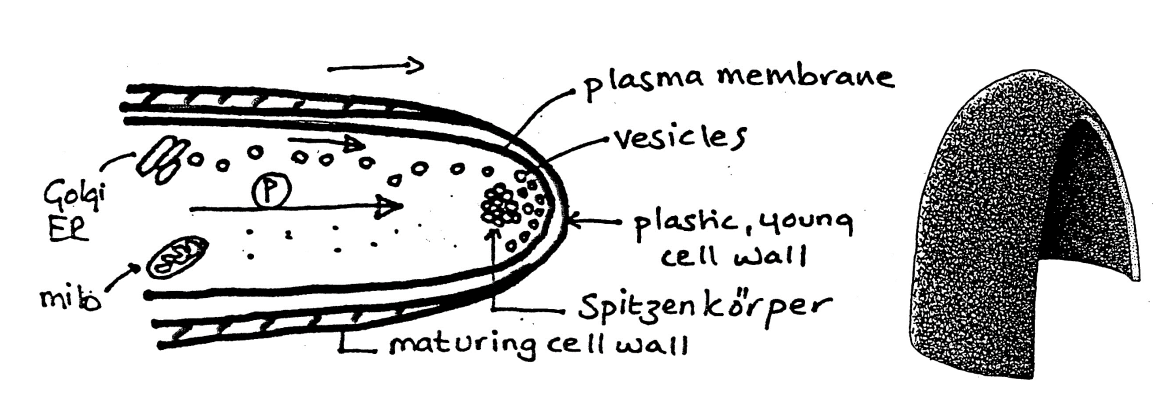
Generalised model for polar growth: Step 3
Vesicles fuse to the apical plasma membrane
exocytosis
deliver the cargo to the area to
where the nascent wall is thin and deformable
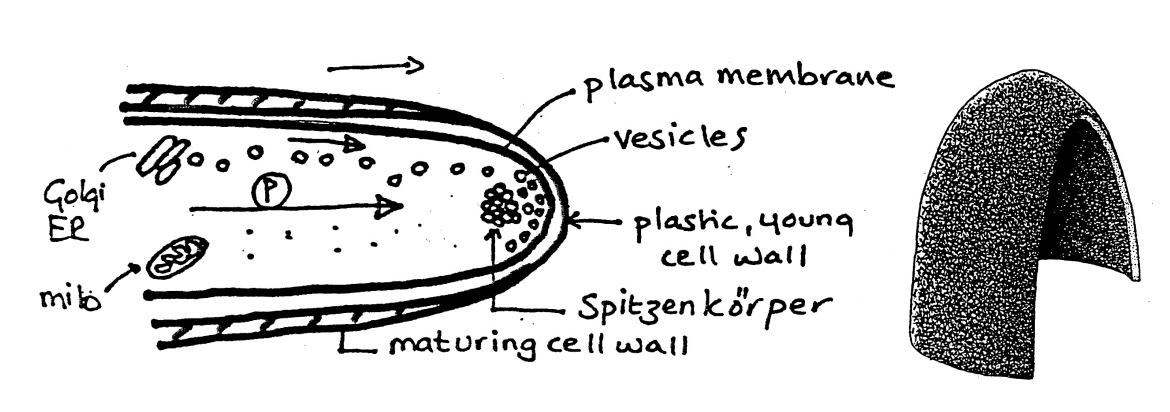
Generalised model of polar growth: Step 4
Cell wall synthesising enzymes
secreted as zymogens
Hydrostatic pressure “pushes” out the tip
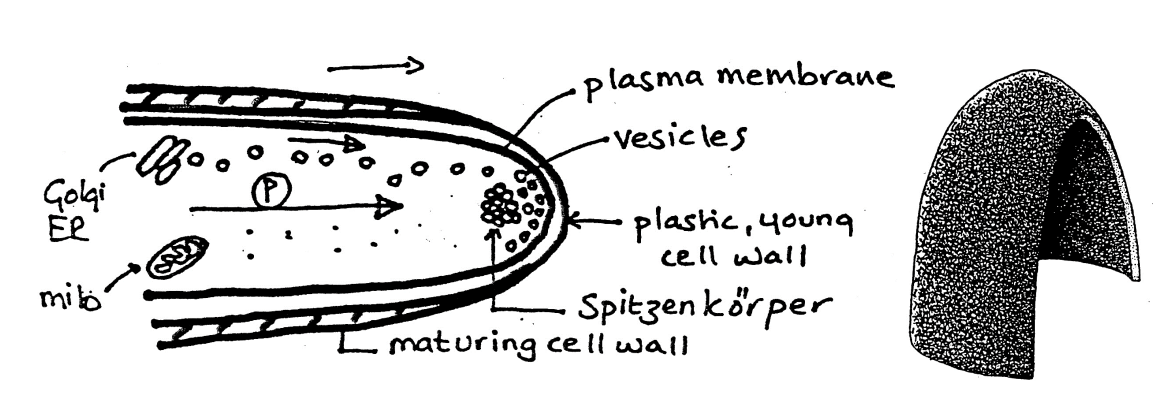
Generalised model of polar growth: Step 5
The wall matures to form a rigid, layered lateral wall
Why is control of this system needed
achieved regular shape
regular growth rate 1mm/hour
directional changes much centre on targeted exocytosis
Where are vesicles found
Densely packed in the tip
forms Spitzenkorper
vescile organisation centre
If disrupted
fungal growth ceases
Involvement of Ca2+ ions with actin and exocytosis
Apical plasma membrane Ca2+ permeable ion channels permit localised Ca2+ influx
helps create tip with high [Ca2+]cyt (microM) gradient
High [Ca2+]cyt stimulates exocytosis
maintains actin in F-actin (filamentous) form
F-actin gives the cytosol mechanical strength
without high viscosity
![<ol><li><p>Apical plasma membrane Ca2+ permeable ion channels permit localised Ca2+ influx</p><ul><li><p>helps create <strong>tip</strong> with high [Ca<sup>2+</sup>]<sub>cyt</sub> (microM) <strong>gradient</strong></p></li></ul></li><li><p>High [Ca<sup>2+</sup>]<sub>cyt</sub> stimulates exocytosis</p><ul><li><p>maintains actin in F-actin (filamentous) form</p></li></ul></li><li><p>F-actin gives the cytosol mechanical strength</p><ul><li><p>without high viscosity</p></li></ul></li></ol><p></p>](https://knowt-user-attachments.s3.amazonaws.com/3947143a-de98-4e09-8a05-99cf273dfd43.png)
How are vesicles directed?
SNARE proteins help move them to specific fusion sites
Cognate pairs of SNAREs exist in vesicles and target membrane
have been found in animal and yeast cells
Ustilago maydis
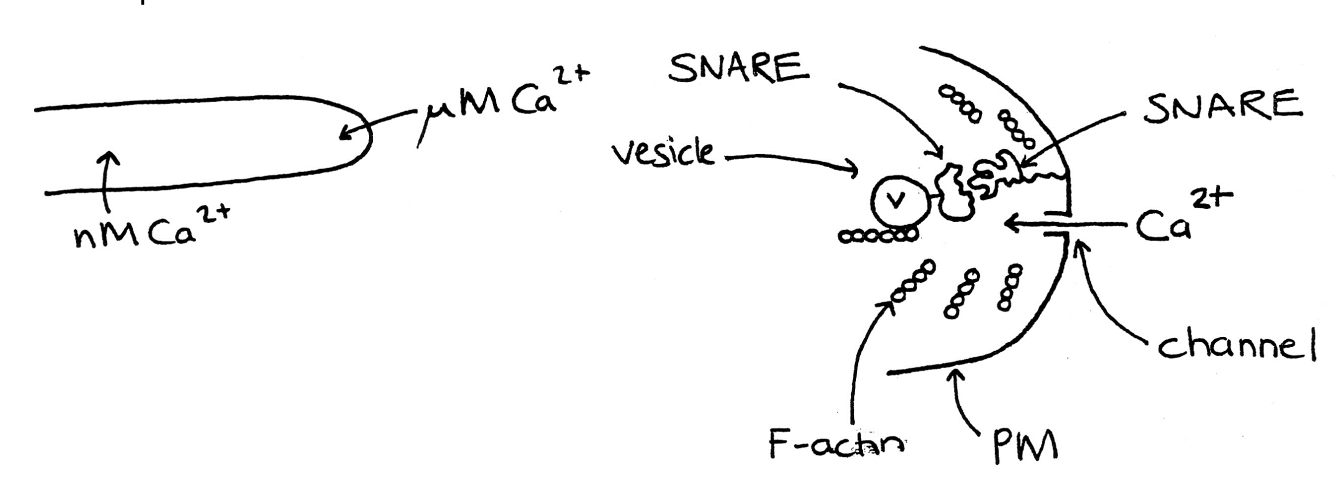
What is the tip also used for?
Exoenzyme secretions
Used to degrade insoluble external substrate
cellulose, lignin, chitin
So it can be absorbed
Where secreted?
Some small molecules
from the lengths (depends on wall porosity)
Larger
secreted at the nascent tip
When do hyphae secrete exoenzymes
When need to grow
adaptive and economical
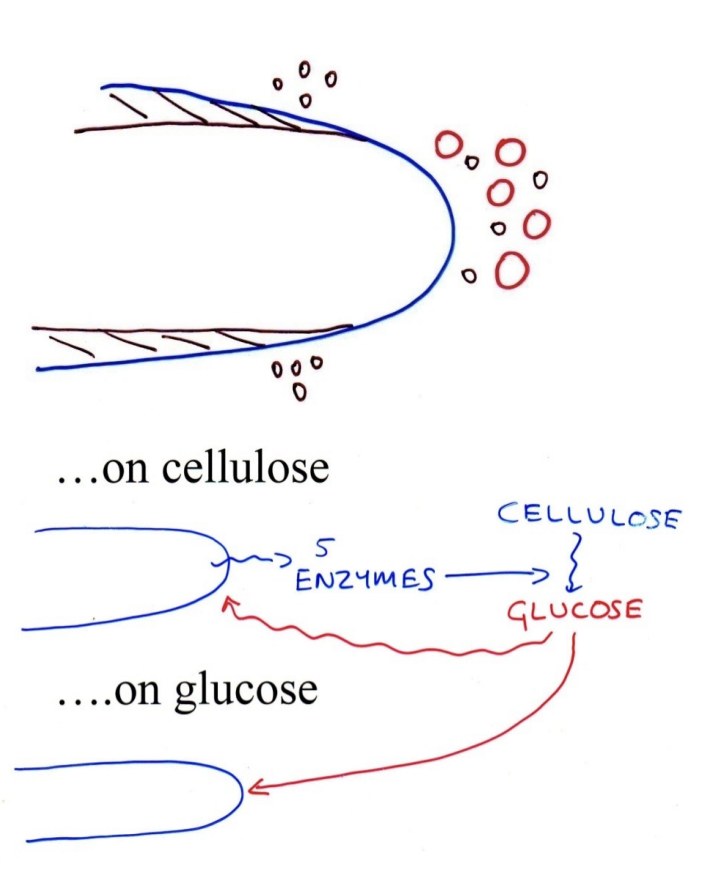
Catabolite repression
Can grow Trichoderma with cellulose and only C source
secretes enzymes
breaks it into glucose
But
Grow it on glucose
production of enzymes inhibited
Catabolite repression
In all fungi
Why have catabolite repression
Use the nutrition source that is in greatest abundance
Switch off metabolism that becomes unnecessary
less energy wasted
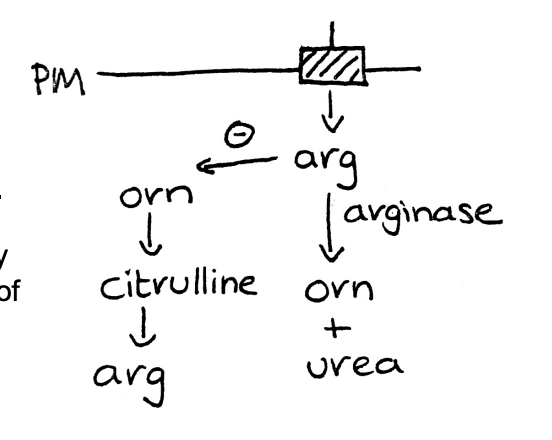
E.g of catabolite repression: Neurospora
Grow on no arginine
Makes own: anabolic reaction
made from ornithine
Supply with arginine
arginine inhibits enzymes that make ornithine
so no more arginine is made
arginase activity in cell increases
Catabolic production
makes arginine→ ornithine
Taking up materials (once digested etc)
Needed for new materials for growth
Fungal growth could be infinite
Many transport proteins
e.e yeast has 19 hexose transporters
Many of the transport proteins
Fungu run on proton economy
Many pumps are P-type A+-ATPase
In plasma membrane
encoded by PMA1 and PMA2
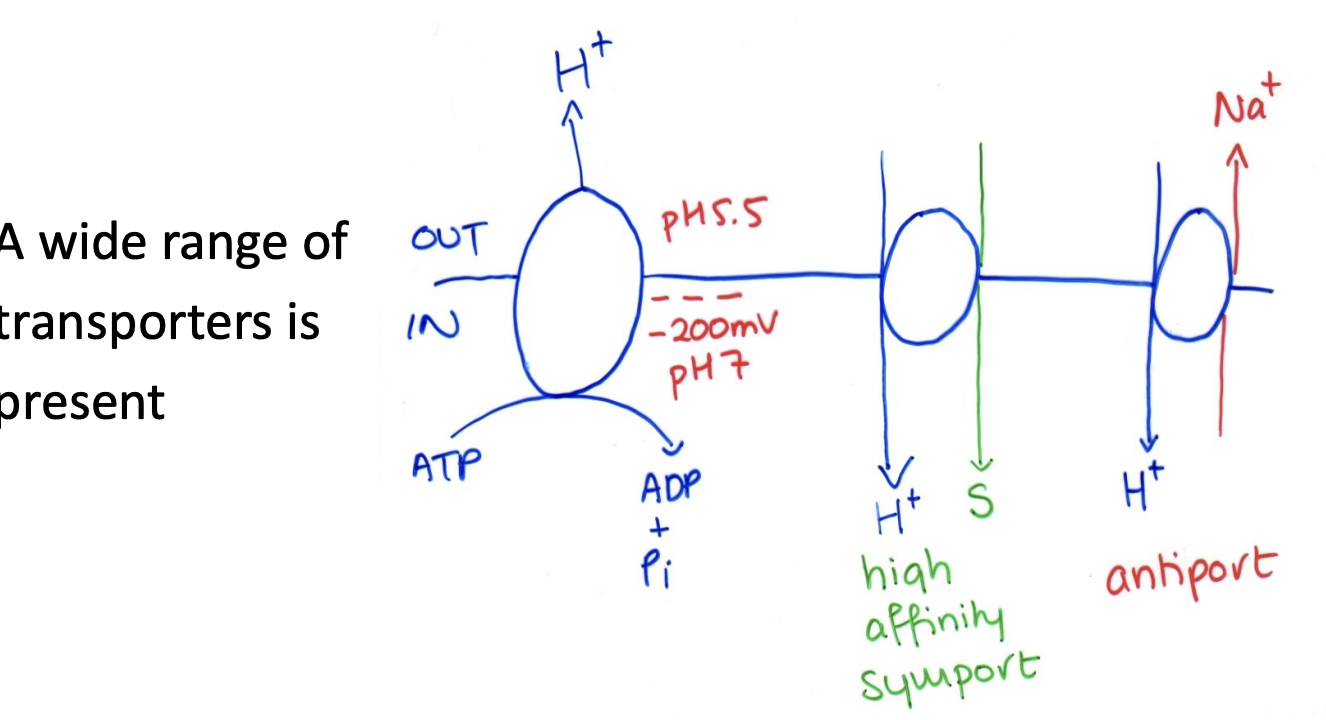
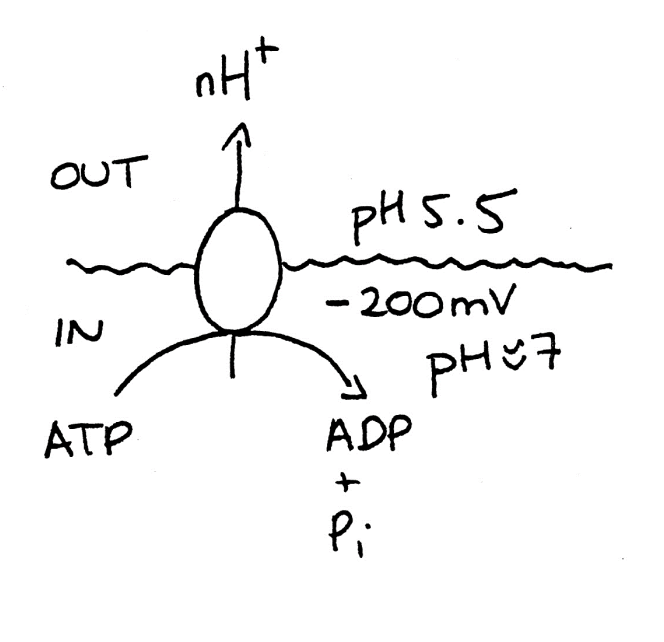
What H+ pumps do
uses 50% of fungal ATP to set up
proton electrochemical potential graidnet
Drives H+ coupled nutrient uptake
symport
or
Explusion of potentially toxic ions
(e.g SOD2 Na+/H+ antiporter in pombe)
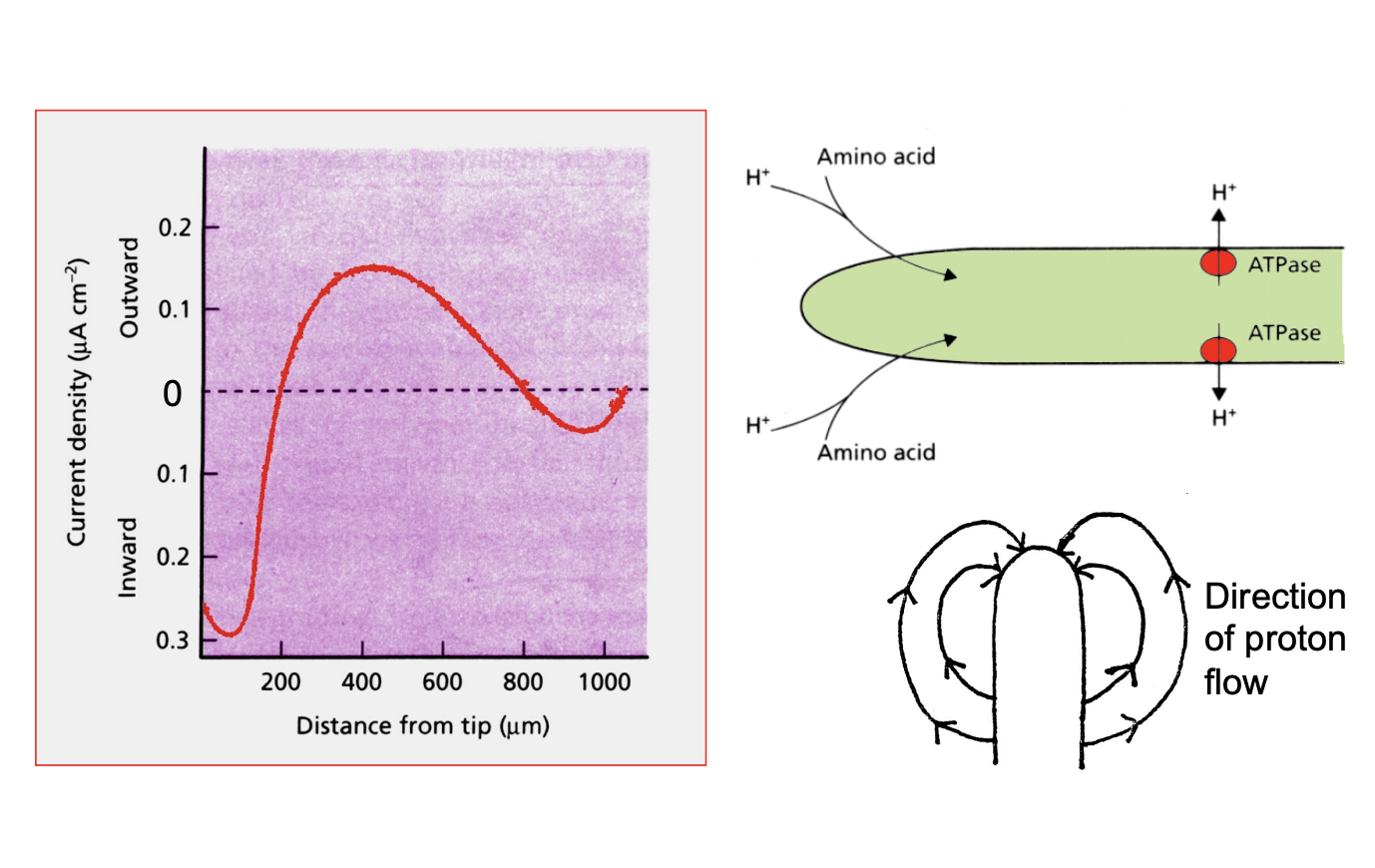
What do experiments show sabout H+ arounf the hyphae
Use vibrating microeletrode experiments
Show extracellular H+ circuit around growing hyphae
acts as a DC power supply
H+ coupled nutrient symporters at the apical membrane act as resistor in the circuit
Points of re-entry
indicate nutrient absorption
at and just behind the apex
Disappear in absence of nutrients
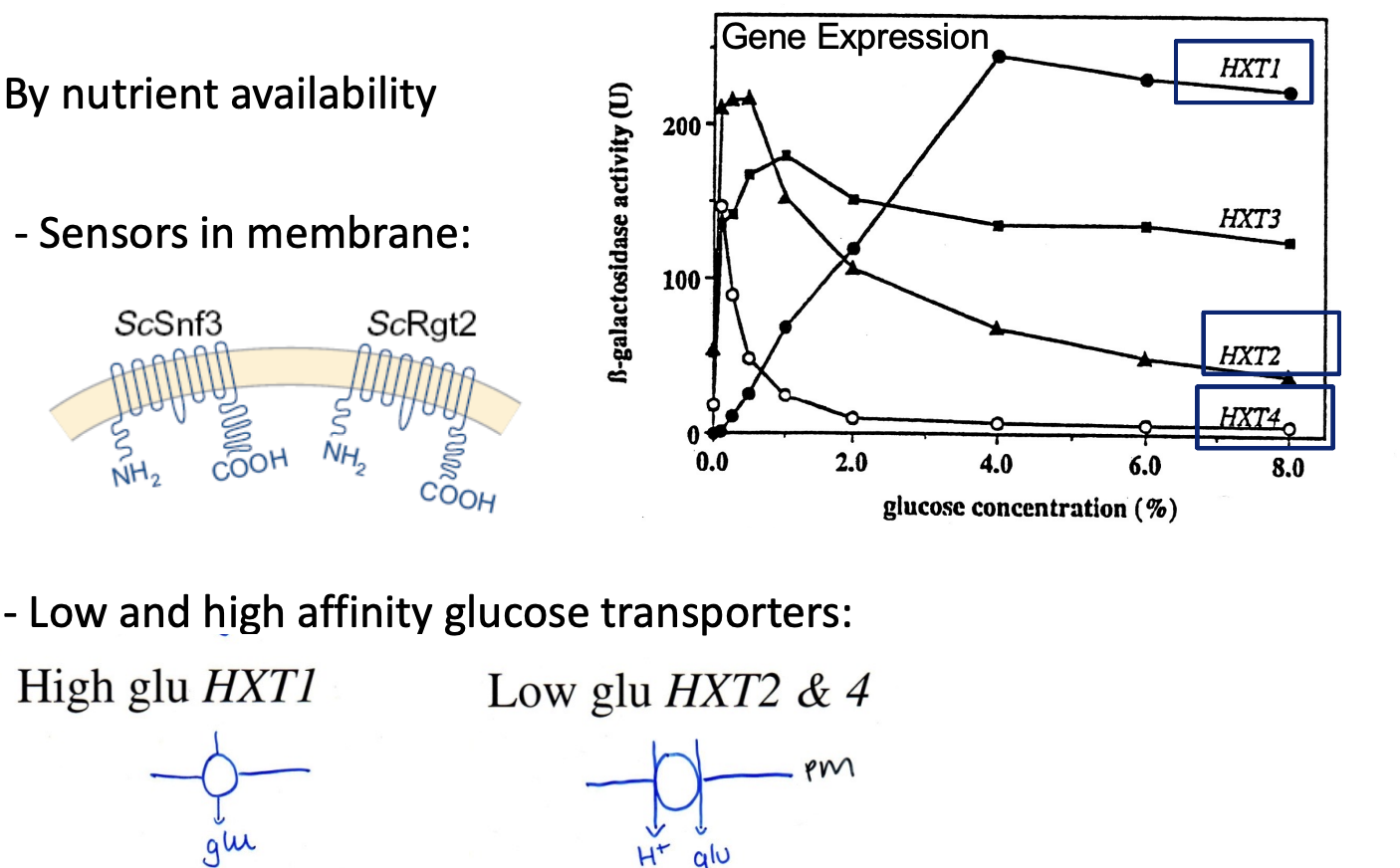
Expression of nutrient uptake transporters
Tightly regulated
as metabolic enzymes
Example of transport regulation: Saccharomyces cerevisiae
HXT expression- hexose transporter
At low external glucose
HXT2 and HXT4 are expressed
high affinity H+ coupled glucose sympoters
entry of glucose triggers phosphoylation (via cAMP?)
of Regulatory C-terminus of plasma membrane H+-ATPase
Activit increase to aid the H+ coupled glucose symporters
Other genes encode for low affinity non-coupled transporters
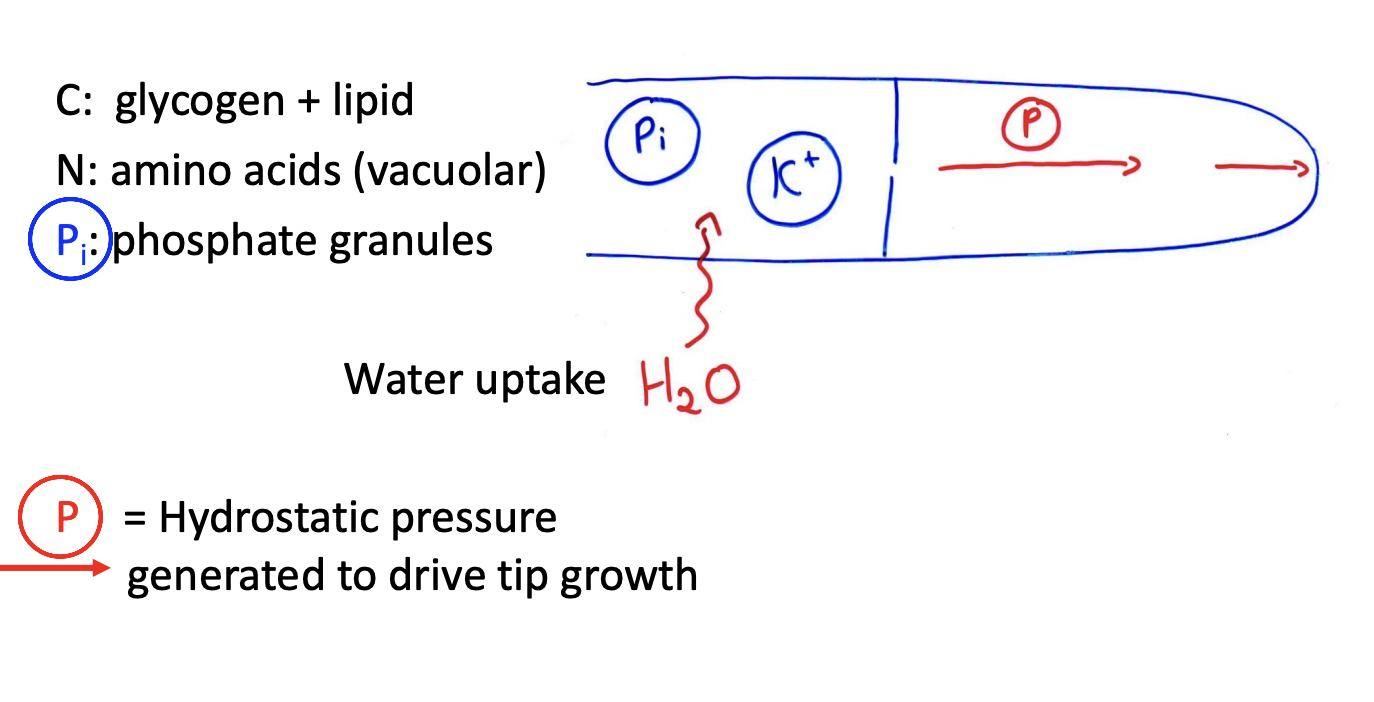
Storage zones
In the older sub apical region
Excess C is stored as:
Glycogen
lipid
Vacuoles
excess N: in vacuolse
ions such as K+
When storaged stuff is needed
Mobilised for growth
What storage zones also do
Generate osmotic gradient
ensure water uptake to generate hydrostatic pressure
needed for growth
What this means
Nutrient uptake and extension:- INEXTRICABLE LINKED
Senescence zone
Older regions are not wasted!
Vacuoles release hydrolytic enzymes
autolysis
Adjacent younger regions absorb breakdown products
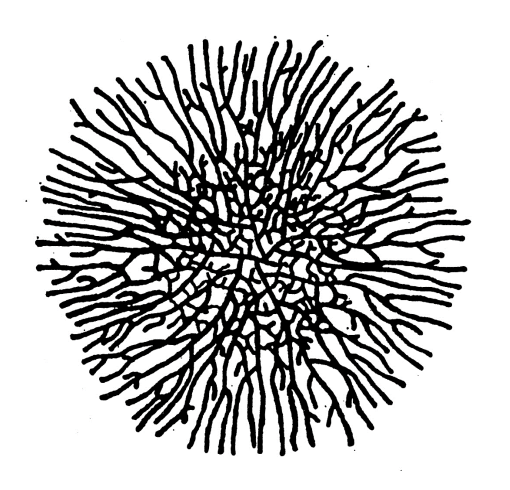
Colony strucuture
Newly emergent hyphae near maximum extension rate
Branch will form
Makes new hyphae
Lateral branches continue to form
at exponential rate
grow at the tip and absorb nutrients
Why are many fungal hyphae autotropic?
So that it can make sure it grows in new environment, even if it doesn’t have any food available yet??
How hyphae colonise uncolonised substatum
senses presence of other hyphae
makes sure to grow away from it
So grows into place where not colonised yet$
Mycelium
Hyphae network
Fungal conidiation (spore formation) regulated by…
Circadian clock

Mycelium advantages
efficient at maximising nutrient uptake from substratum
Make sure to not grow in rubbish places
sets up nutrient depletion zones beneath the colony
when depletion is acute:
growth in these regions is switched off
growth only in leading esge mycelium
It is out there foraging
Advantage of concentrating growth capacity at the leading edge
Not limited to rate of diffusion
out there foraging
unlike:
yeast and bacterial colonies
rely on diffusion
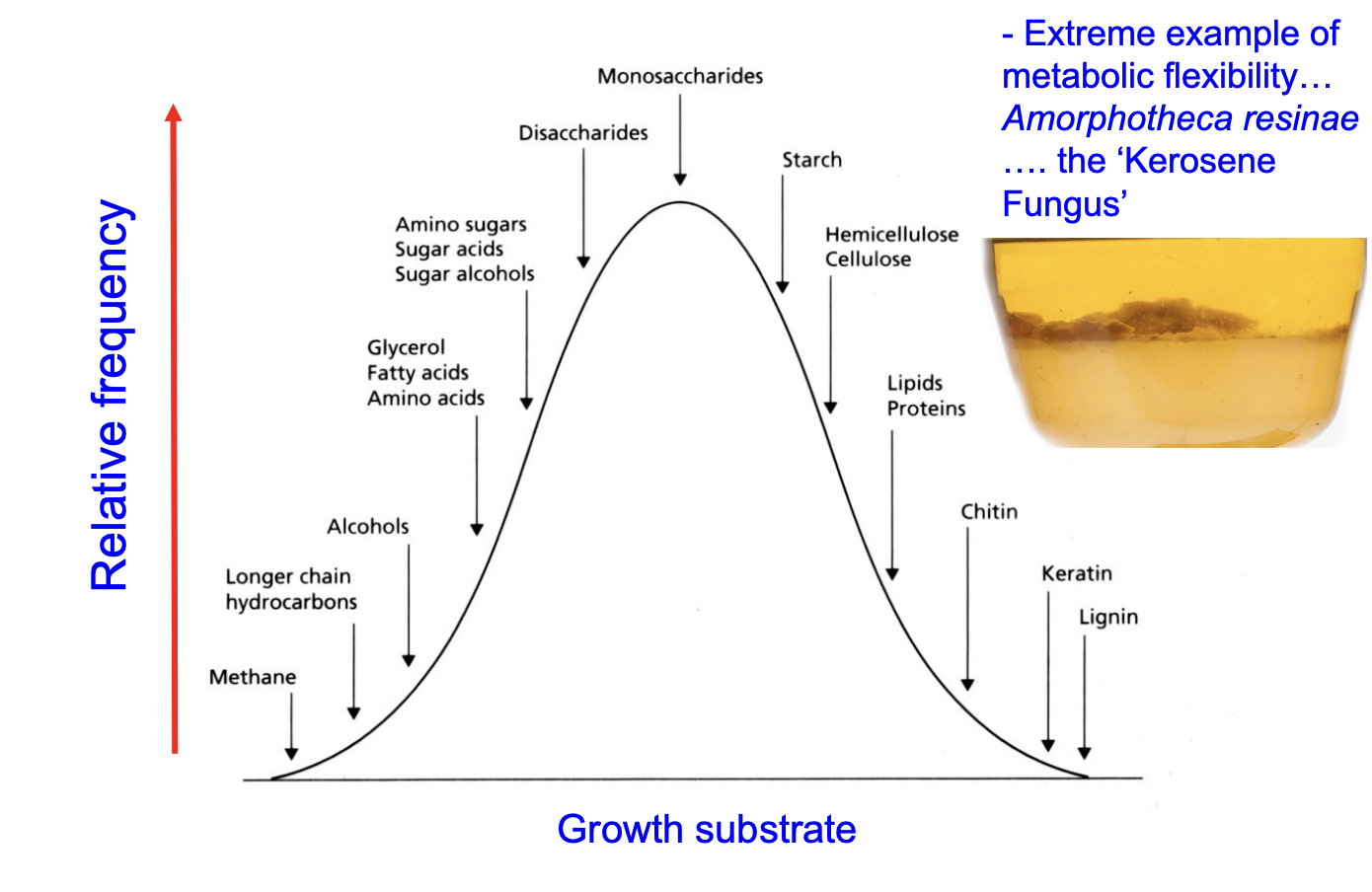
Metabolic flexibility: vast range of growth substrates
Metabolic flexibility: Glycoxylate cycle
Under low C conditions (e.g when fungus is phagocytoses by macrophages)
Glycolate cycle switched on
Phagosome is nutrient poor environment
Fungus upregs expression of enzymes of glyoxylate cycle
Diverts TCA
into gluconeogenesis for production of hexoses for growth
C.glabrata strain carrying mutation in isocitrate lyase genes
Are non-pathogeneic
Therefore: glyoxylate cycle imporant for
Pathogenesis
Metabolic flexibility: Oxygen
Majority of fungi are aerobes
but
some are obligate anaerobes
niche habitats
cow rumen
no longer have mitochondria

Why useful to be facultative anearobe
Increases survival chances:
TCA is switched off
mitochondria production suppressed
glycolysis relied upon for ATP
ethanol end-product may be metabolised as a C source when an oxygen supply returns