Topic 5: Seawater Chemistry
1/83
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
84 Terms
Covalent bonds
Share electrons
Ionic bonds
Give/take electrons
2 reasons why water is a polar molecule
Difference between size of H and O atoms causes bend in geometry of water molecule
Negative and positive charges at either end = polarity
How hydrogen bonds are created in water
Water is a molecule with positive and negative ends, it attracts particles with negative and positive ends – like other water molecules
Cohesion
Water attracts water; surface tension (hydrogen bonds)
Adhesion
Water ‘sticks’ to surfaces (electrostatic bonds)
Why water is the “Universal solvent”
Because it is polar and so dissolves other molecules with negative and positive ends (like salts)
Thermal properties of water (and why they are important)
Water stores heat energy
Important for ocean circulation + other processes
3 phases of water
Solid (ice)
Liquid water
Gas (water vapour)
Process of water going from solid to liquid
Melting
Process of water going from liquid to gas
Vapourization
Process of water going from gas to liquid
Condensation
Process of water going from liquid to solid
Freezing
How energy changes with water’s phases
Use energy to create bonds (i.e liquid to solid), remove energy as we break bonds (i.e solid to liquid)
Kinetic energy
Energy an object has because of its motion, with a faster object having more (water in all states has kinetic energy)
Heat
Heat is energy; amount of energy transferred from one body to another due to temperature differences; proportional to average kinetic energy
Temperature
A number, way to measure the average kinetic energy (response to addition/removal of heat)
Calorie
The amount of heat required to raise the temperature of 1 gram of water by 1 degree C
Characteristics of water in solid state (ice)
Molecules vibrate
Stay in same position
Characteristics of water in liquid state (water)
More kinetic energy
Bonds starting to break
Characteristics of water in gaseous state (water vapour)
Most kinetic energy
All bonds broken
Heat capacity definition
Heat required to raise the temperature of 1 gram of a substance by 1 degree C
Heat capacity of water
Pure water has a very high heat capacity (1.oo cal/gram/degree C)
Application of heat capacity to water
Water can absorb (or release) large amounts of heat while changing relatively little in temperature (takes a lot of time for water to heat up or cool down)
Ocean heat content definition
Total amount of heat stored by oceans— the large ocean has a large heat storage capacity meaning the atmosphere has not warmed as rapidly as it could (due to climate change)
% of Earth’s heat stored by ocean
More than 90% percent of the Earth’s extra heat (since 1955, even with 2x uptake since 1993)
Consequences of oceans absorbing so much heat
Oceans being pushed to the edge of their capacity
Changes in ocean circulation
Changes in ocean ecosystems
Changes in ocean climates
Why sea surface temperature is important
Influences weather patterns, storms
Consequences of increasing ocean temperature
Fish metabolism
Nutrient availability
O2 vs CO2 content
Ecosystems
Migration and breeding
Threaten sensitive marine life
Heatwaves in major ocean basins
Amount sea surface temp has risen by
5 degrees C
Latent heat
When water undergoes a change of state a large amount of heat is absorbed or released
Phase Changes and ‘Latent Heats’
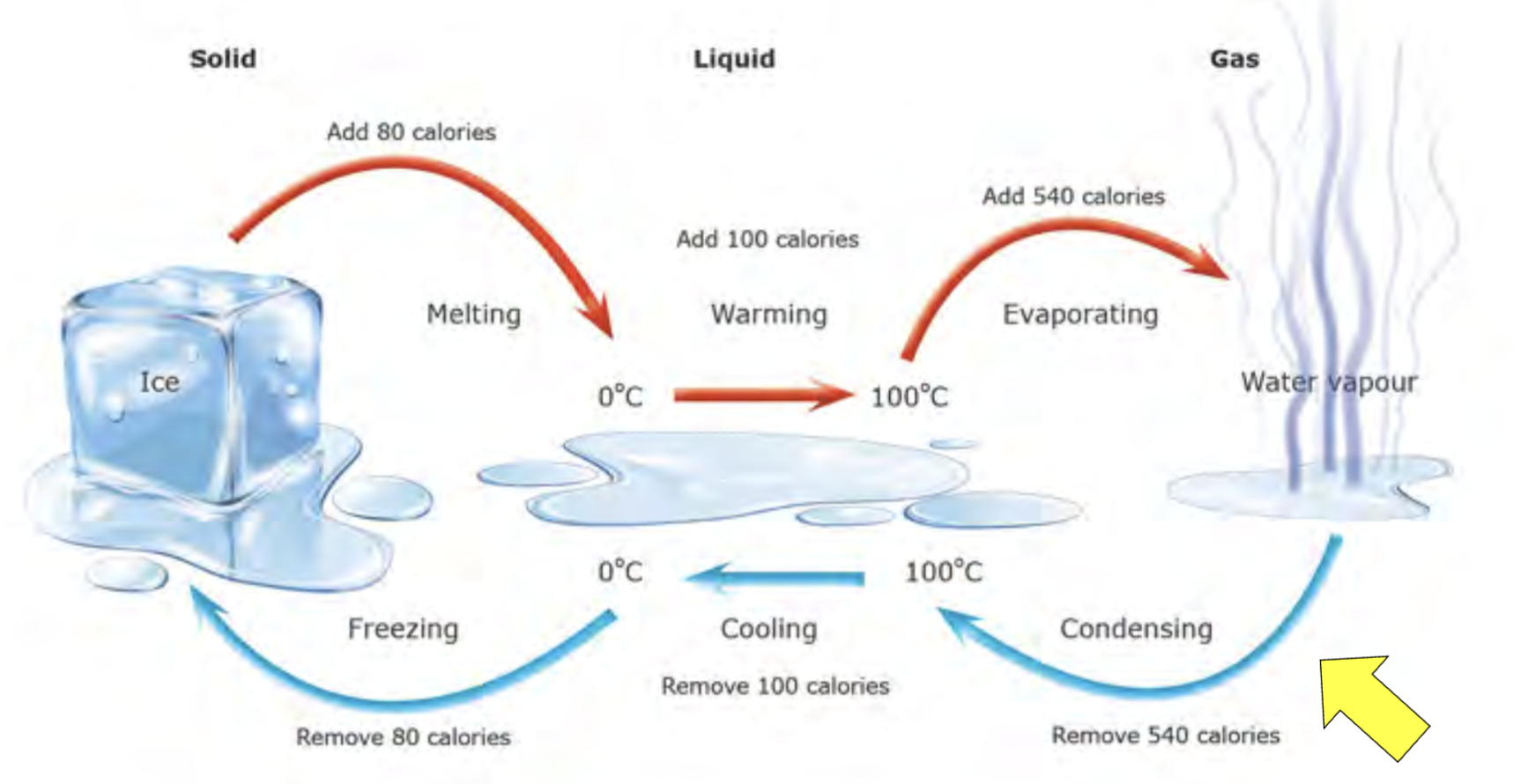
Thermostatic properties
Properties that moderate temperature changes (i.e high ocean heat capacity)
Global Thermostatic Effects
Transfer of heat from the tropics towards the poles, moderates global temperatures
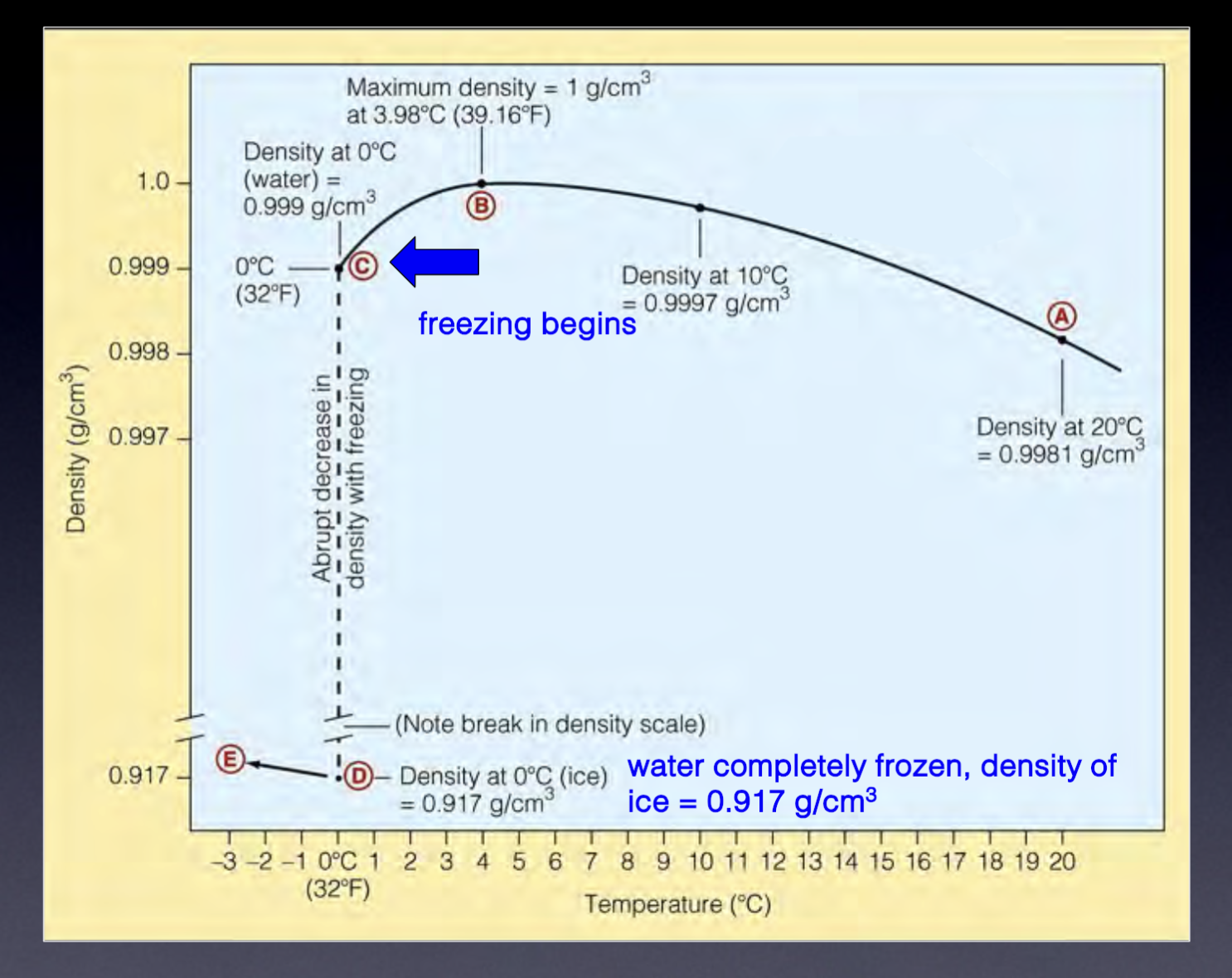
Density of pure water
1.0 g/cm³
How density of water changes at depth
Density increases as heat is removed
Maximum density just before freezing (as ice is less dense than water)
Why ice is less dense than water
Phase change occurs from liquid to solid, rapid movement means liquid expands slightly as more hydrogen bonds (rigid framework) form, therefore ice is less dense and expanding
Density of ice
0.917 g/cm³
Maximum density and temp of water
1.0 g/cm³ at 3.98 degrees C
Percentage of water and dissolved solids in seawater
~96.5% pure water
3.5% dissolved solids
How dissolved solids in seawater change freezing point and latent heat
Reduce freezing point to -1.91 degrees C
Lowers latent heat by ~4%
How salt determines freezing point of water
More salt = lower freezing point
3 reasons to be concerned about salinity of ocean water
Presence of ions and dissolved gases that cause salinity important because:
Make life in ocean possible – needed for some biological processes
Affects ocean circulation
Can provide information about palaeo-oceanic conditions
Salinity definition
Total amount of dissolved solids and gases in water
Concentration of salt in seawater
Averages 3.44% (given as parts per thousand – 34.4‰)
Types of salt in seawater
6 major ions represent 99% of all dissolved solids, Cl- and Na+ dominate
Major gases dissolved in seawater
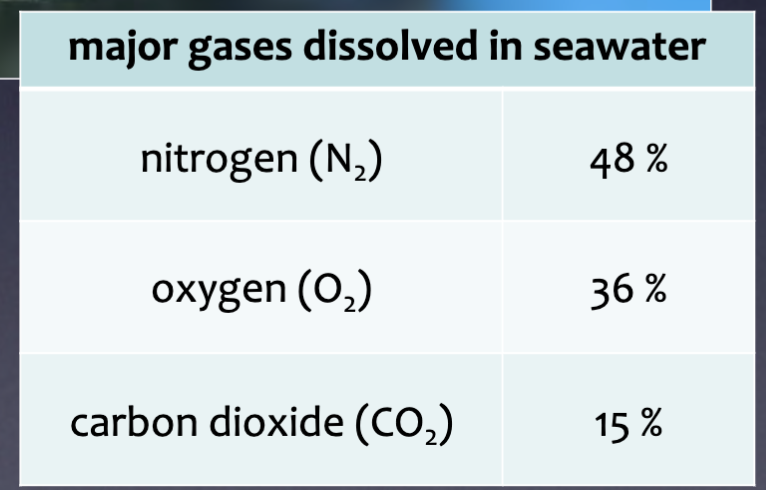
How gases enter seawater
Common atmospheric gases are easily dissolvable in seawater because the ocean is in contact with the atmosphere
Why nitrogen is important in the ocean
Upper layers of ocean are saturated with nitrogen
Required by organisms for protein-
building
Why oxygen is important in the ocean
If you have gills – you need this gas for metabolism
Comes from photosynthetic activity and
atmospheric diffusion
How carbon dioxide enters the ocean
Very soluble in seawater
Seawater is a carbon reservoir
Controls on amount of each gas dissolved in the ocean
Dependent on solubility and saturation of the gas
Solubility
Amount of a dissolved gas that the water can hold under a particular set of conditions (0 degrees C and 1 atm of P)
Saturation
Amount of gas currently dissolved in the water, relative to the maximum possible content
Undersaturated
More gas can dissolve
Saturated/supersaturated
Gas may be released
Why are most atmospheric gases are saturated in the ocean, but O2 and (CO2) are not?
They are rapidly used by living organisms
2 major factors affecting solubility of a gas
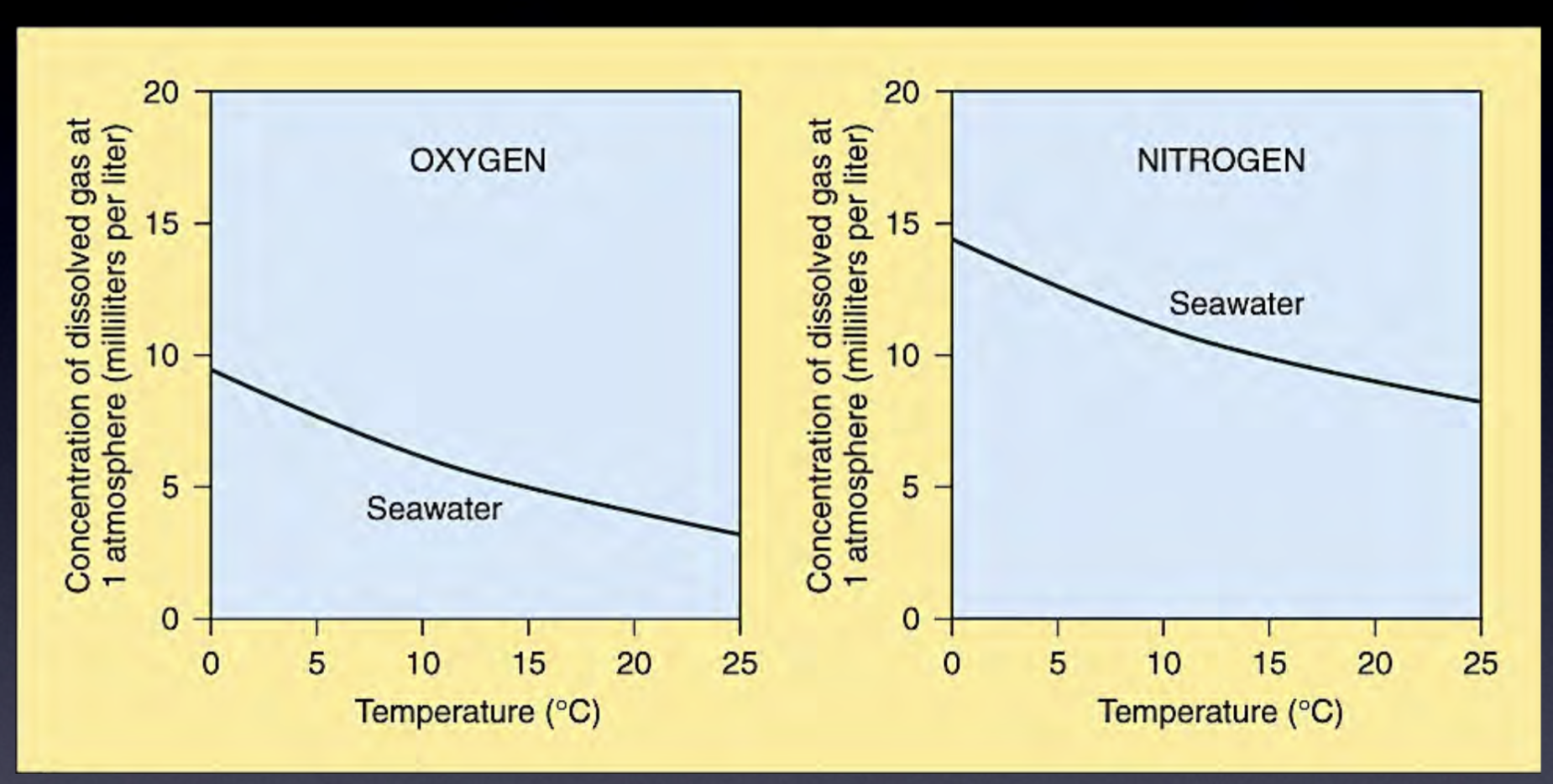
Gas solubility increases with increasing pressure
Also increases with decreased temperature
Why does O2 concentration still decrease in deep ocean?
No photosynthesis (light cannot penetrate)
Reduced atmospheric diffusion
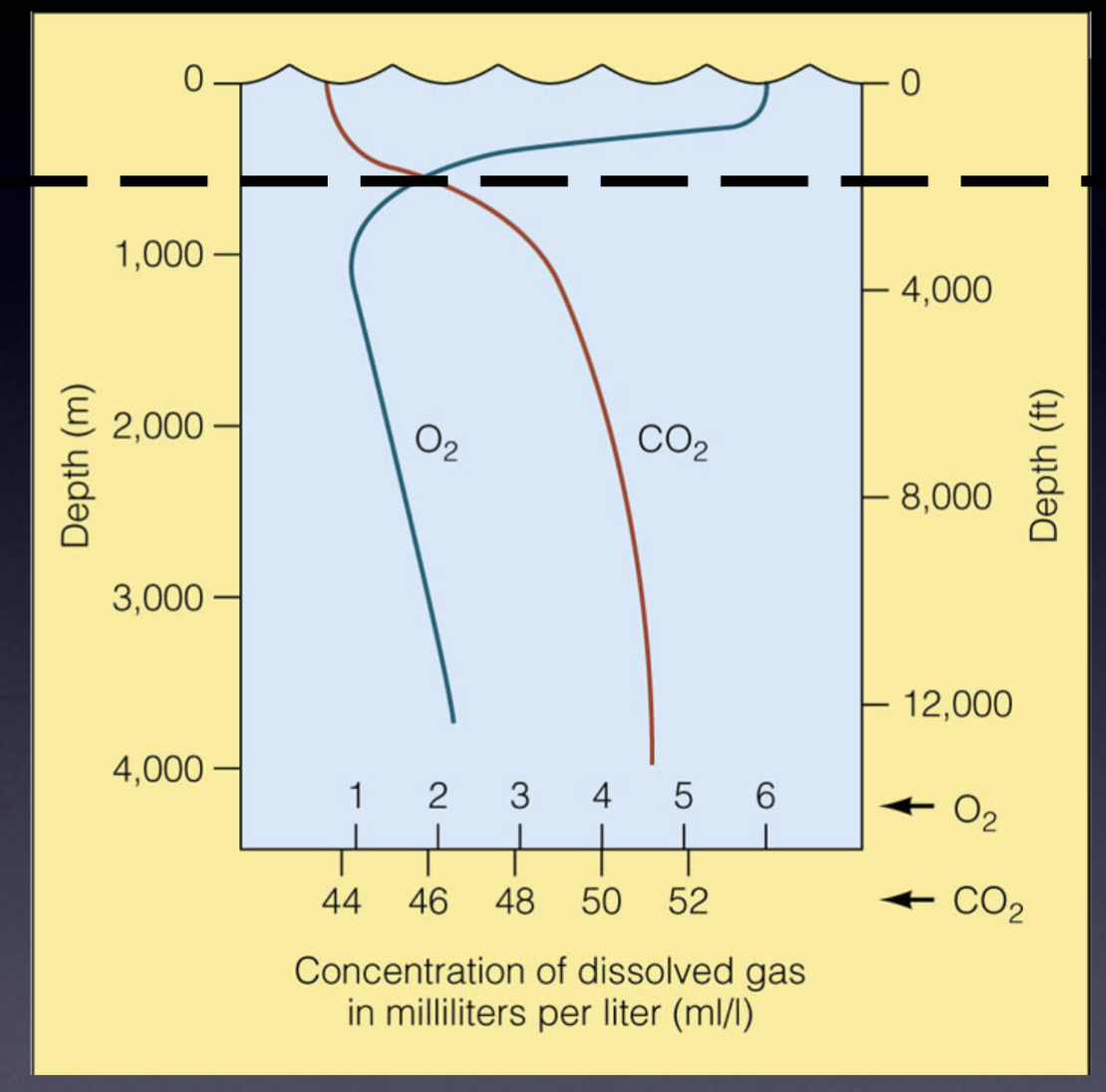
Why does CO2 concentration increase in deep ocean?
CO2 is more soluble with lower temp and higher pressure
No photosynthesis to absorb CO2
OML/OMZ (Oxygen Minimum Layer/Zone)
Around ~ 200 to 1000 m deep in ocean where O2 saturation is at its lowest
Why OML/OMZ forms
No input from atmosphere or
photosynthesis
Output – respiration and
decomposition
No better conditions for O2 dissolution (after this zone, water becomes deeper + colder and more O2 dissolves)
Where salinity varies
On the surface
At depth
Around the globe
Salinometer
Measures salinity of seawater by measuring electrical conductivity
Principle of constant proportions (Forchhammer’s Principle)
Ratio of dissolved solids in the ocean is constant, different salinities but proportions of major ions remains the same (i.e both sites have 55.04% of dissolved solids being Cl- ions)
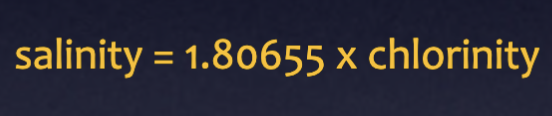
Chlorinity
Measure of Cl- in seawater (most abundant dissolved solid in seawater)
Salinity/chlorinity equation
3 ways ions/compounds enter seawater
River discharge
Volcanic eruptions
Hydrothermal activity at mid-ocean ridge (seafloor spreading)
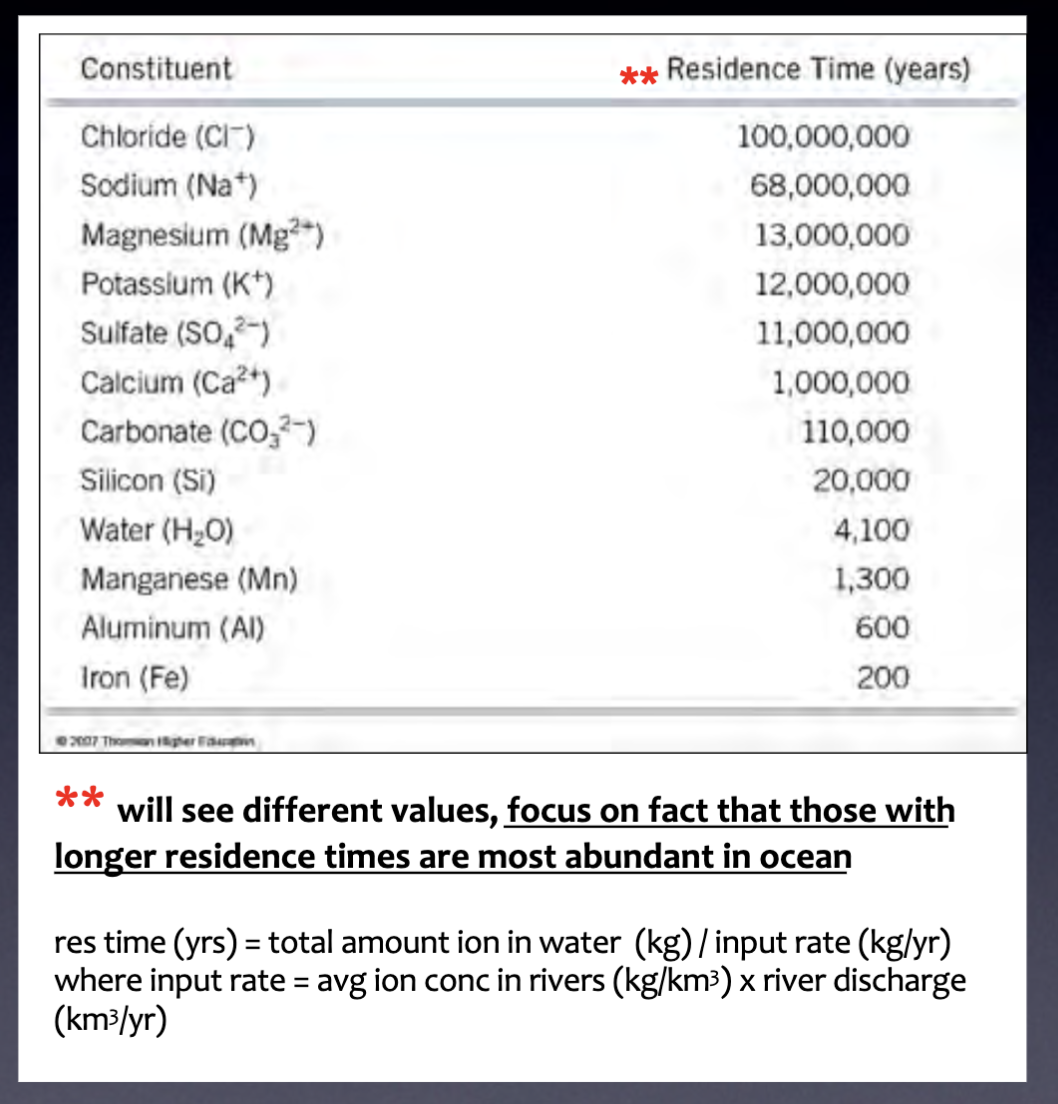
Residence time
Average length of time that an ion spends in the ocean (times vary depending on how chemically active an ion is)
Composition of seawater vs freshwater
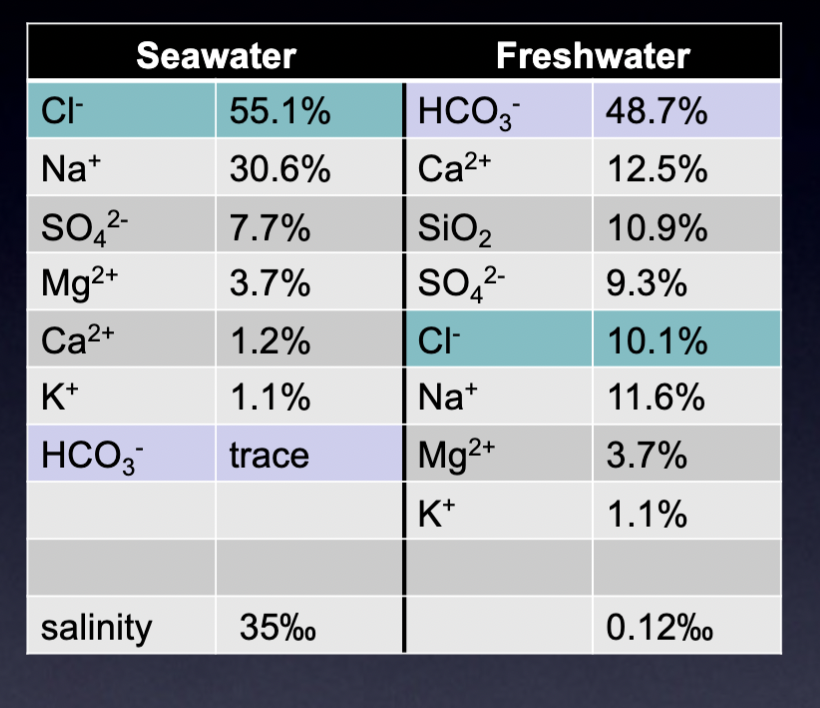
Why the ocean doesn’t get saltier
Because it is in equilibrium— ion removal rate = addition rate
4 ways ions/compounds are removed from seawater
Adsorption and precipitation
Sea spray
Biological processes
Hydrothermal activity at mid-ocean ridge (seafloor spreading)
2 Global salinity trends
Tropics of Cancer and Capricorn – high
evaporation rates (higher salinity)
Poles and Equator – freshwater influx (lower salinity)
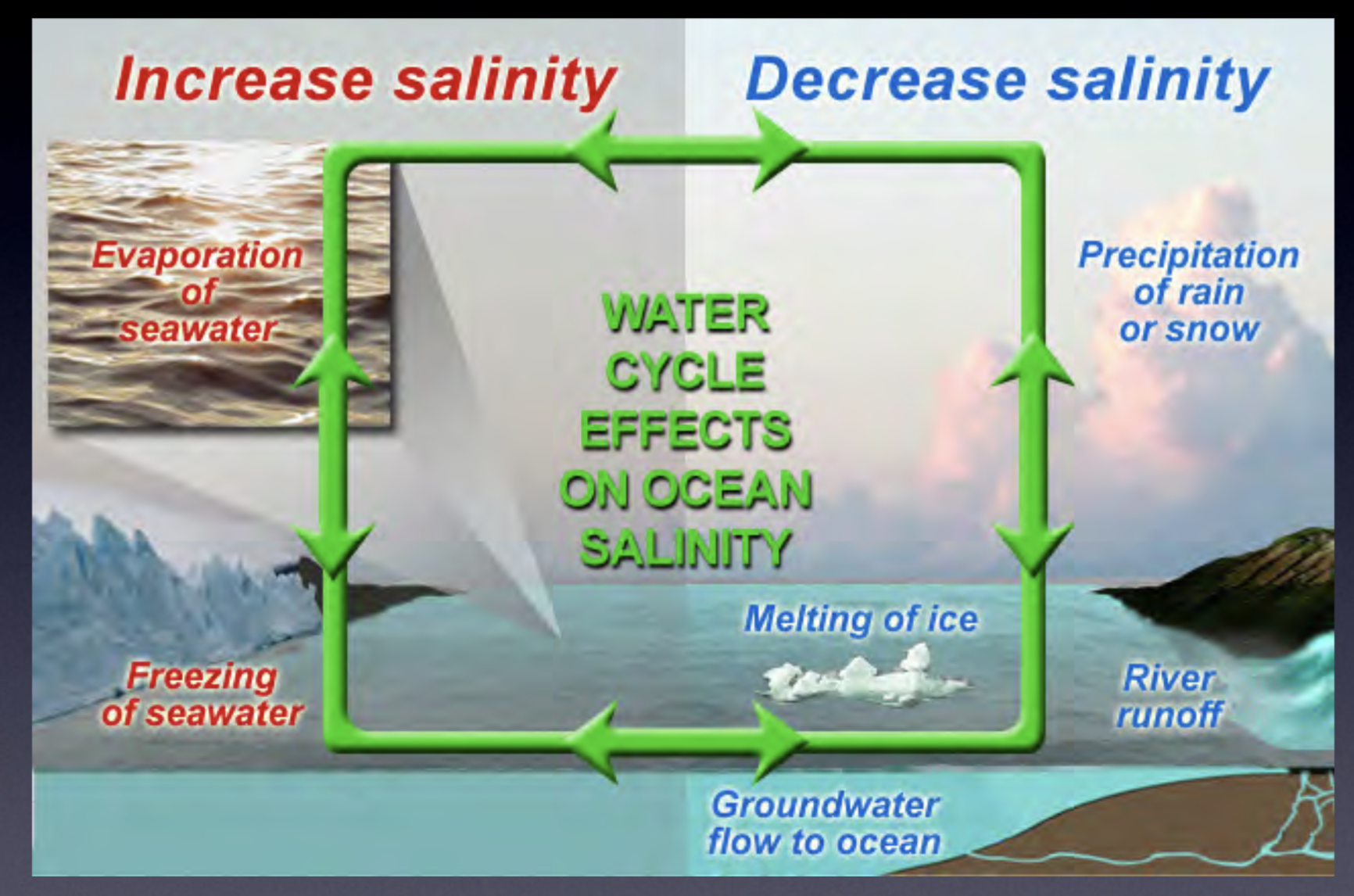
2 ways salinity can increase
Evaporation of seawater
Freezing of seawater
4 ways salinity can decrease
Precipitation of rain and snow
River runoff
Melting of ice (glacier melt)
Groundwater flow to ocean
How to change salinity
Add or remove water (less water = higher salinity)
‘Layered ocean’
Variation in salinity at depth in the ocean
Halocline
Layer of rapidly changing salinity with depth
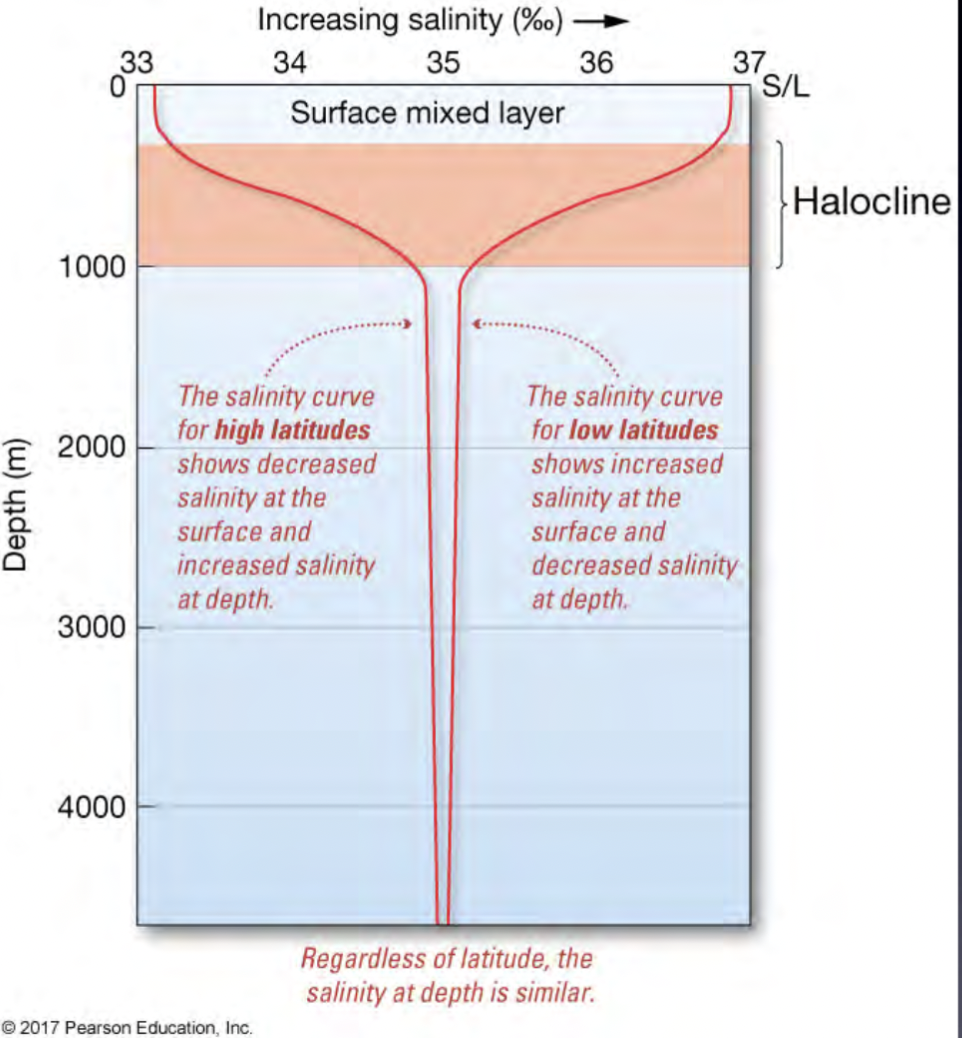
How surface salinity differs across latitude
Higher salinity at surface at low latitudes due to more evaporation
Seawater density
~1.022 to 1.030 g/cm³
4 ways seawater density increases
Increases with greater depth
Increases with greater salinity
Increases with decreased temperature
Increases with increased pressure
3 density zones (water masses)
Surface zone (2% of ocean water)
Pycnocline (18% of ocean water)
Deep zone (80% of ocean water)
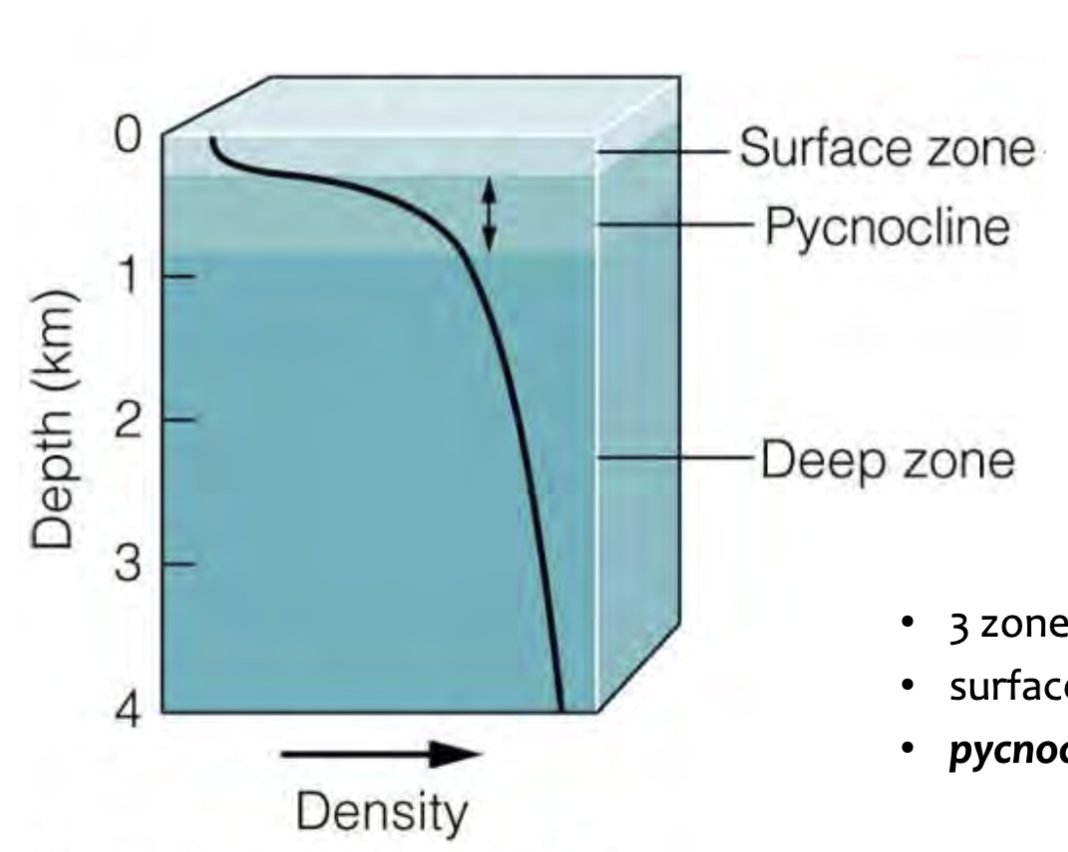
Pycnocline
Layer of rapidly changing density (between surface and deep zones)
Pycnocline vs Thermocline
Same thing! Rapidly changing density = rapidly changing temperature (because temp is greatest influence on density)