Solubility
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
What is solubility?
The amount of something that can be dissolved in solution.
Why is solubility important?
Electrolytes, nutrient molecules and drugs need to show adequate solubility.
Gases involved in respiration must be soluble in tissue fluids.
What is the relationship between gas solubility and pressure?
Likewise with temperature.
Gas solubility increases with pressure.
Think of how a coke bottle, if closed, doesn’t appear to be carbonated, but if you open it, bubbles appear.
As temperature increases, solubility of gases decreases. As temperature increases, gas molecules gain more energy and escape from the liquid phase into the gas phase more easily.
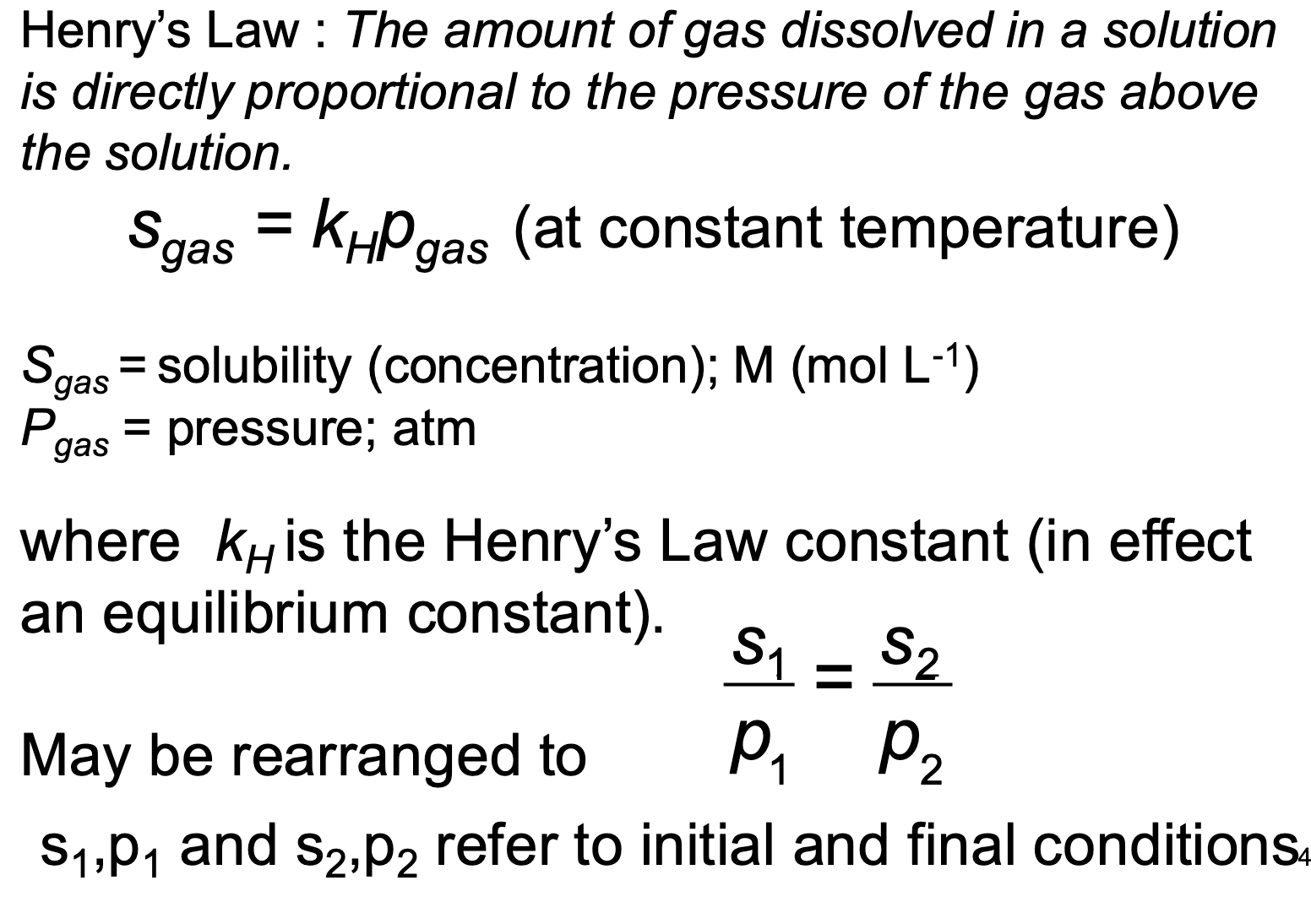
What is Henry’s Law?
The larger the value of kH, the more soluble the gas is.
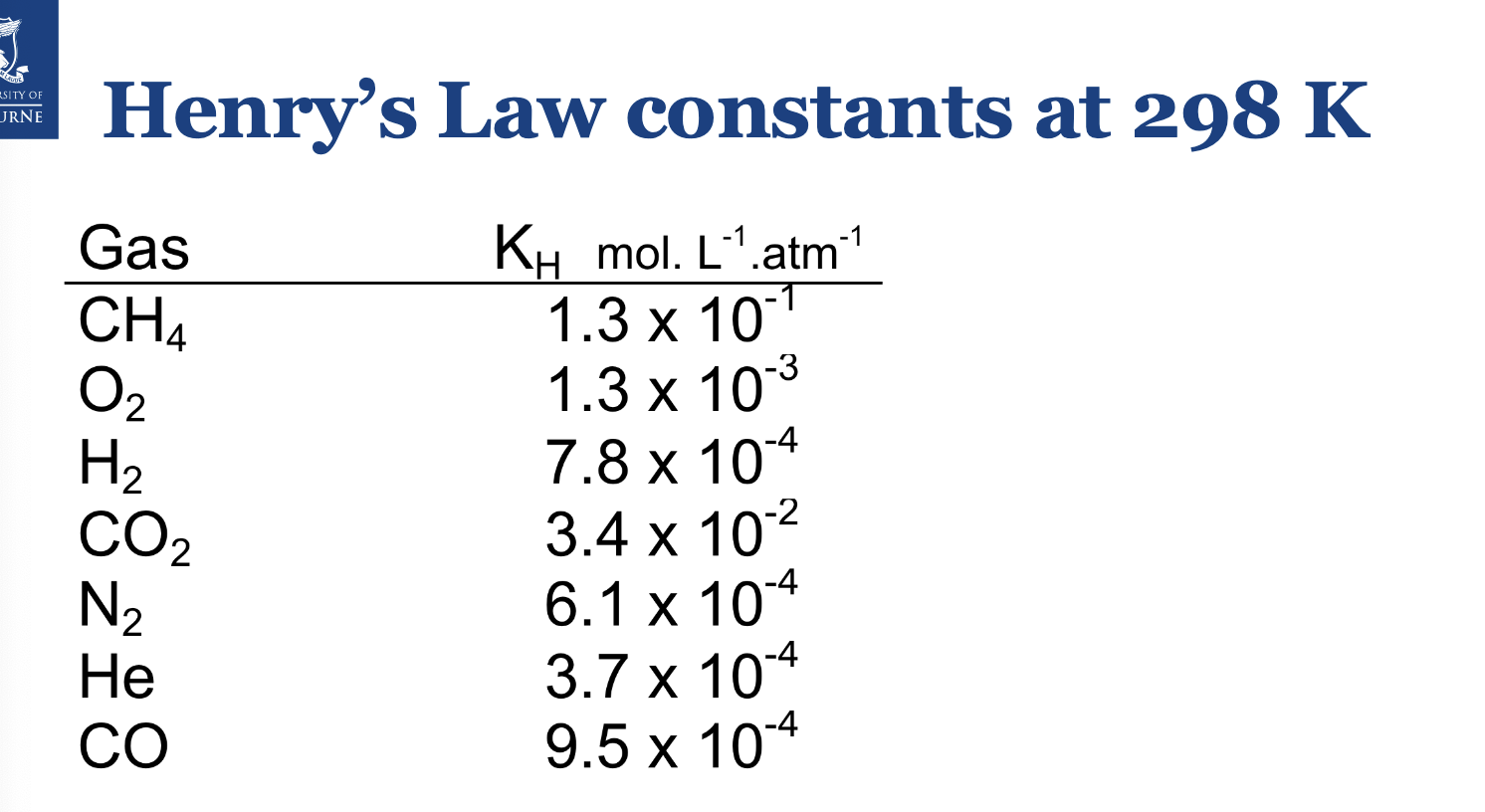
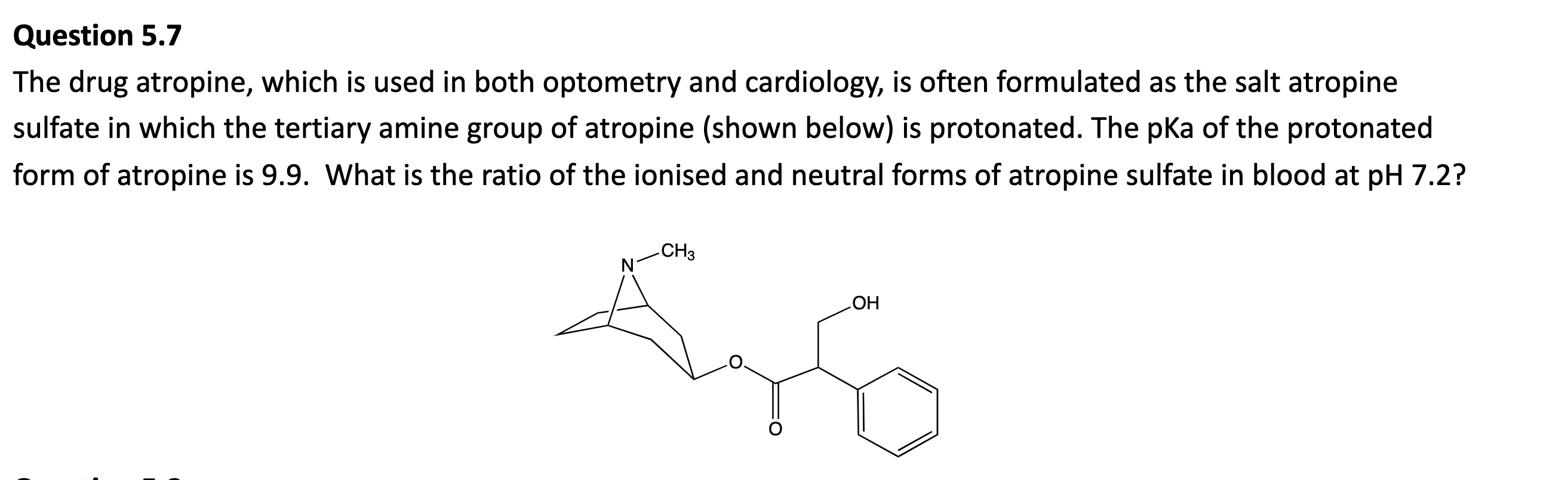
If the concentration of carbon dioxide dissolved in a can of soda is 0.075 M, what is the partial pressure of carbon dioxide gas in the can at 25ºC?
Sgas=kHPgas
At 25ºC, kH of CO2 = 3.4×10-2
Pgas=0.075/3.4E-2=2.20atm. It’s atm as the constant units are at atm.
Calculate the solubility of carbon dioxide in water at 0ºC and 3.00 atm pressure when the solubility of carbon dioxide at 0ºC and 1.00 atm is 0.348 g/100 mL
s1/p1=s2/p2
0.348/1=s2/3
s2=0.348×3=1.004g/100mL
What is the effect of temperature on the solubility of ionic solids?
Generally, the solubility of solids increases with temperature (not always).
You can dissolve sugar easily in hot water.
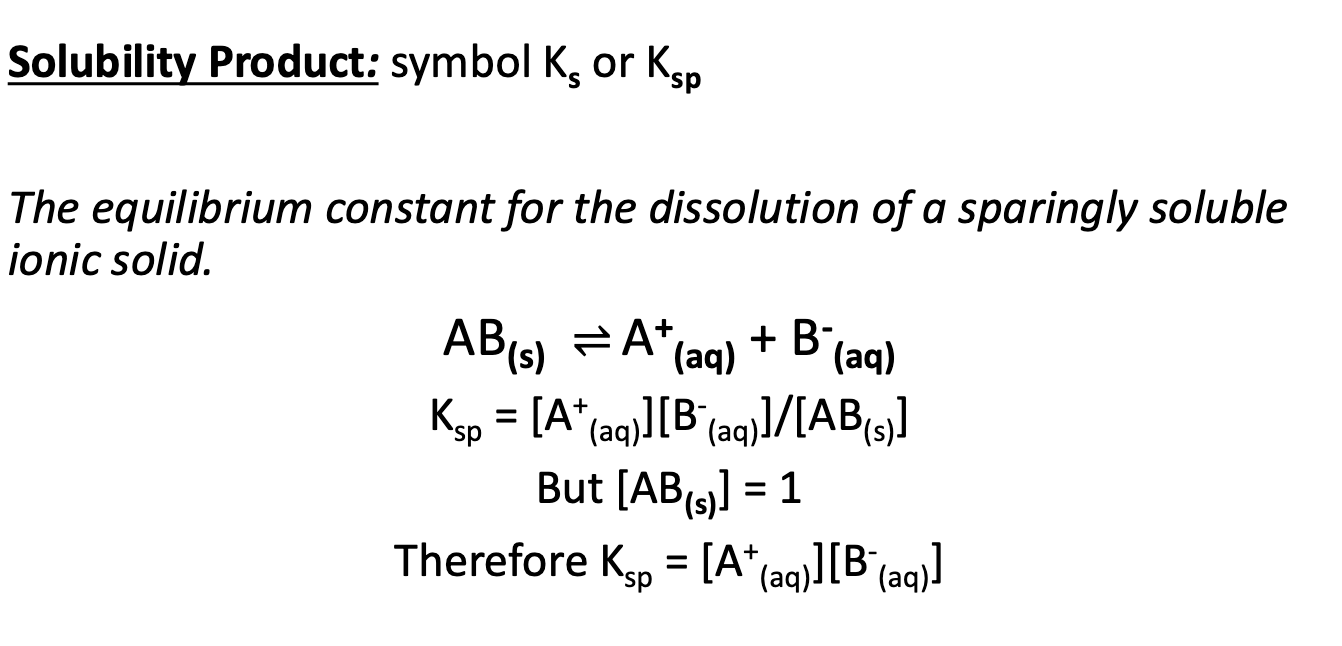
What is the difference between solubility product and solubility?
Solubility = amount of material which dissolves.
Solubility product = equilibrium constant. Gives us an idea of how far the reaction proceeds, but doesn’t tell us the concentration of such components.
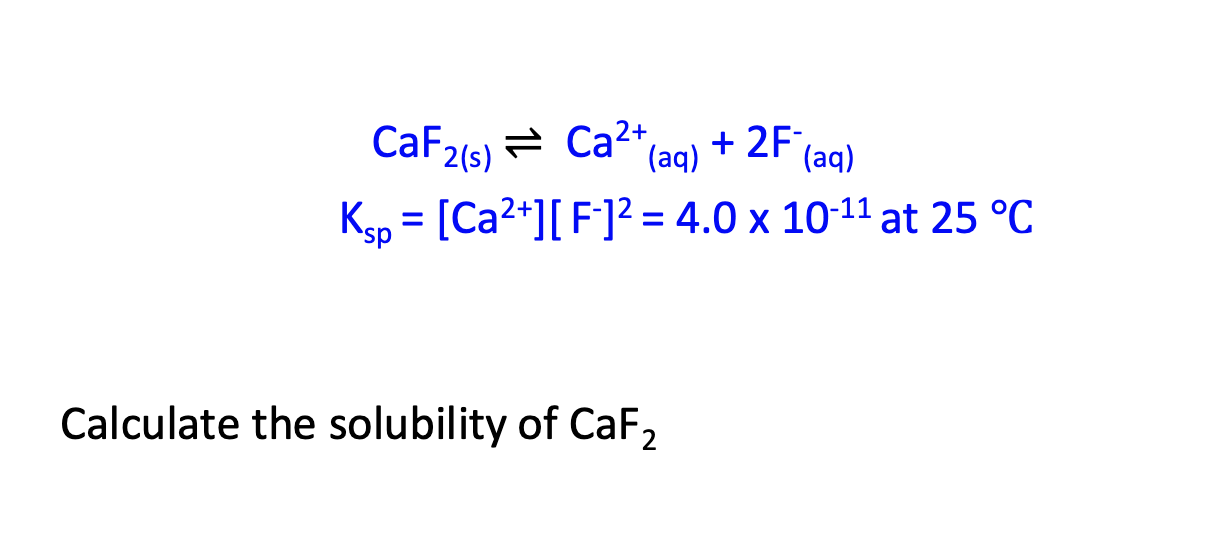
Ksp is very small, thus CaF2 is not very soluble.
Denote concentration [Ca2+] and [F-]2 as s
4.0×10-11=s*(2s)2
4.0×10-11=4s3
s=2.15×10-4M
Think of 2s in an ICE table, where the change wouod be +2s, if initial is 0.

Bi2S3 <→2Bi3++3S2-
Ksp=[Bi3+]>2>[S2-]3
=[2.0×10-15]*[3.0×10-15]³
=1.08×10-13
What is the common ion effect?
Basically LCP, where if you add [products], it will push the reaction back, increasing [reactants], decreasing solubility.
In terms of pH, check image.
![<p>Basically LCP, where if you add [products], it will push the reaction back, increasing [reactants], decreasing solubility.<br><br>In terms of pH, check image. </p>](https://knowt-user-attachments.s3.amazonaws.com/05903482-96e4-4c02-8d0d-724f715ec168.png)
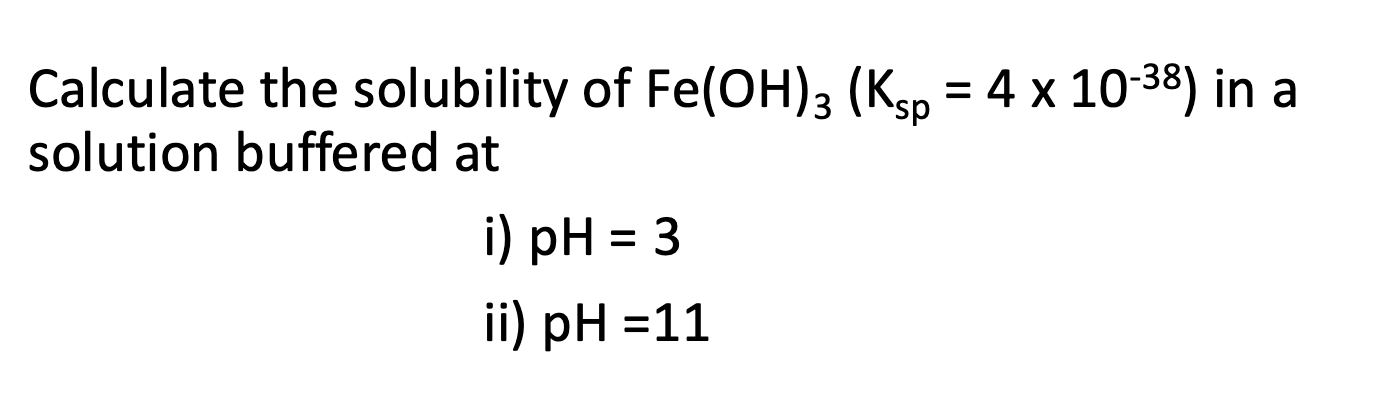
For pH=11, pOH=3
[OH-]=10-3M
![<p>For pH=11, pOH=3<br><br>[OH-]=10<sup>-3</sup>M</p>](https://knowt-user-attachments.s3.amazonaws.com/844e33b3-3a24-4ea5-85ec-1ac8090f5e01.png)
How do you identify if a precipitate will form?
Calculate Qsp.
If Qsp>Ksp, then equilibrium position will shift to the left, leading to the formation of. a precipitate.
If Qsp<JKsp, then no precipitate.


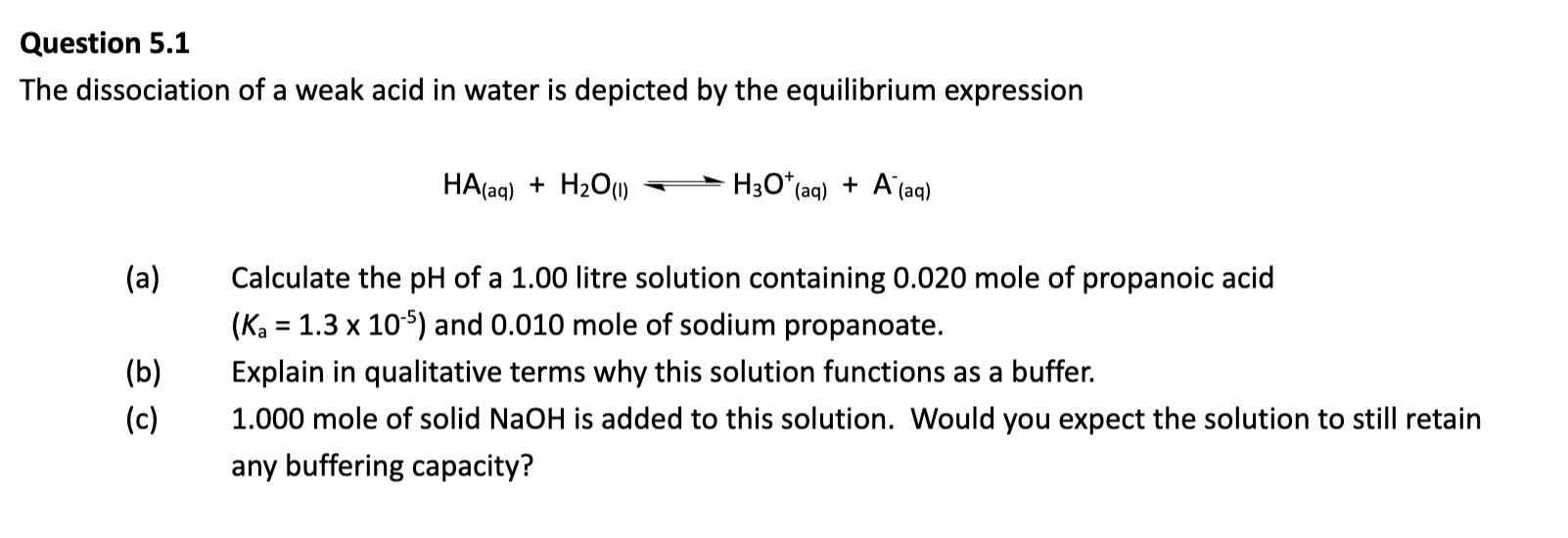

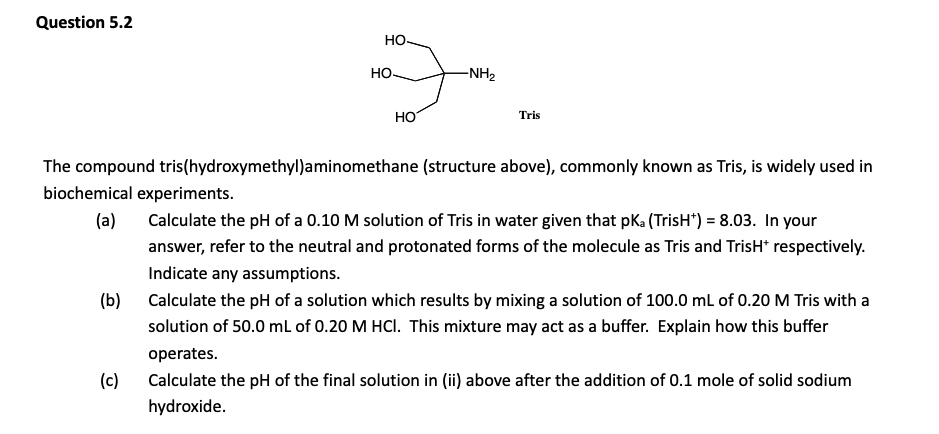



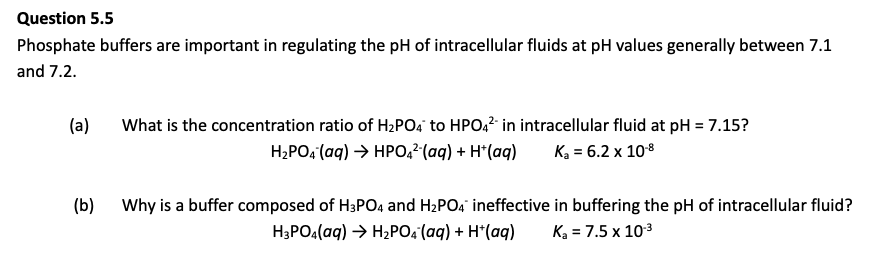
LITTLE H3PO4 TO RESIST CHANGE IN pH as it struggles to resist changes in [H+] at that pH.
If pH ≫ pKa → almost all HA has lost its H⁺ → mostly A⁻ → can't neutralize bases well.
If pH ≪ pKa → almost all is in the HA form → can't neutralize acids well.
AKA for first dotpoint, donates H+ ions to partially oppose the high pH, but not enough HA.
![<p>LITTLE H3PO4 TO RESIST CHANGE IN pH as it struggles to resist changes in [H+] at that pH.<br><br>If <strong>pH ≫ pKa</strong> → almost all HA has lost its H⁺ → mostly <strong>A⁻</strong> → <strong>can't neutralize bases well</strong>.</p><ul><li><p class="">If <strong>pH ≪ pKa</strong> → almost all is in the <strong>HA</strong> form → <strong>can't neutralize acids well</strong>.<br><br>AKA for first dotpoint, donates H+ ions to partially oppose the high pH, but not enough HA.</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/f841a5d1-98b3-4271-a2bc-eaa259b2772e.jpg)

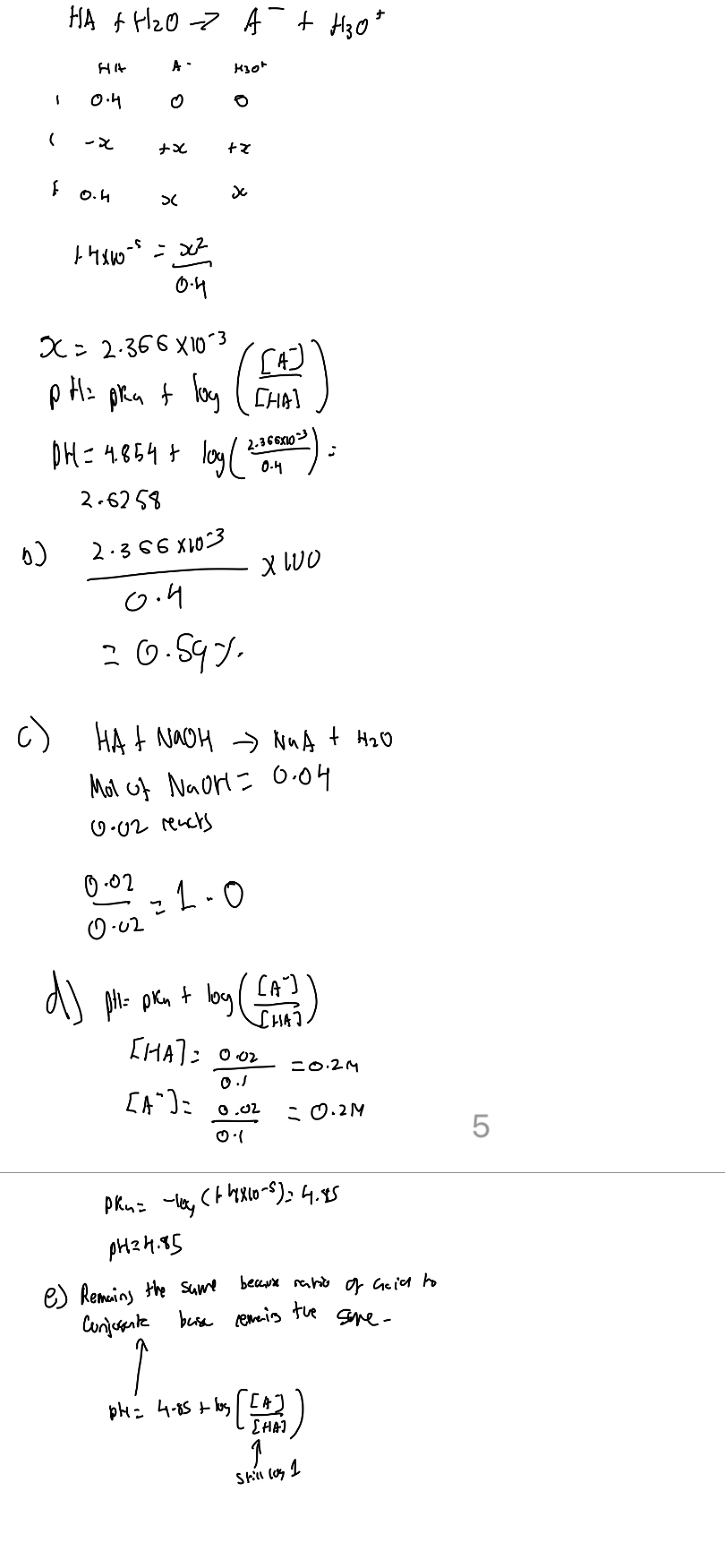
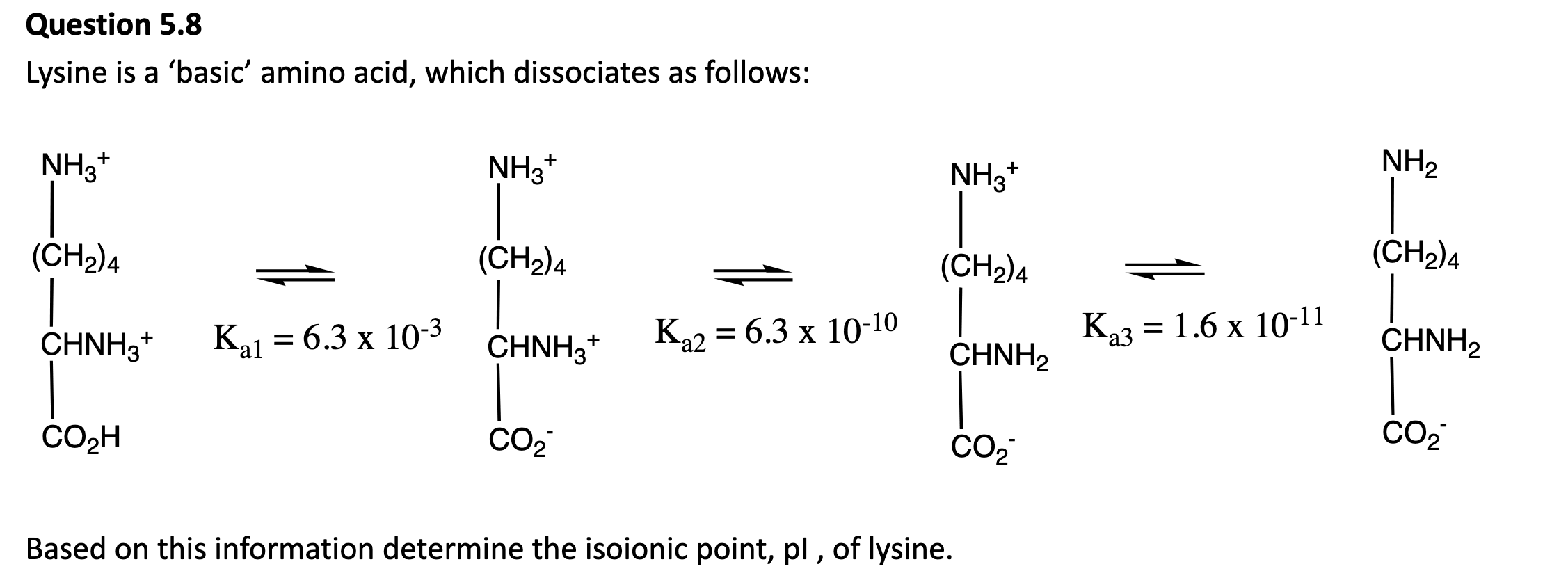
The isoelectric point (pI) is the pH at which the amino acid has no net charge. This occurs when the molecule exists in its zwitterionic form.
Looking at the diagram, that is inbetween Ka2 and Ka3.
pl=average of the pKa values of the zwitterionic forms.
9+10.5/2= 9.75
At 25 °C, the molar solubility of Ag3PO4 is 1.8 × 10-5 mol L-1. Calculate Ksp for this salt.
Ag3PO4 → 3Ag+ + PO43-
(3s)³(s)=Ksp | s=1.8 × 10-5 mol L-1
s=2.834×10-18
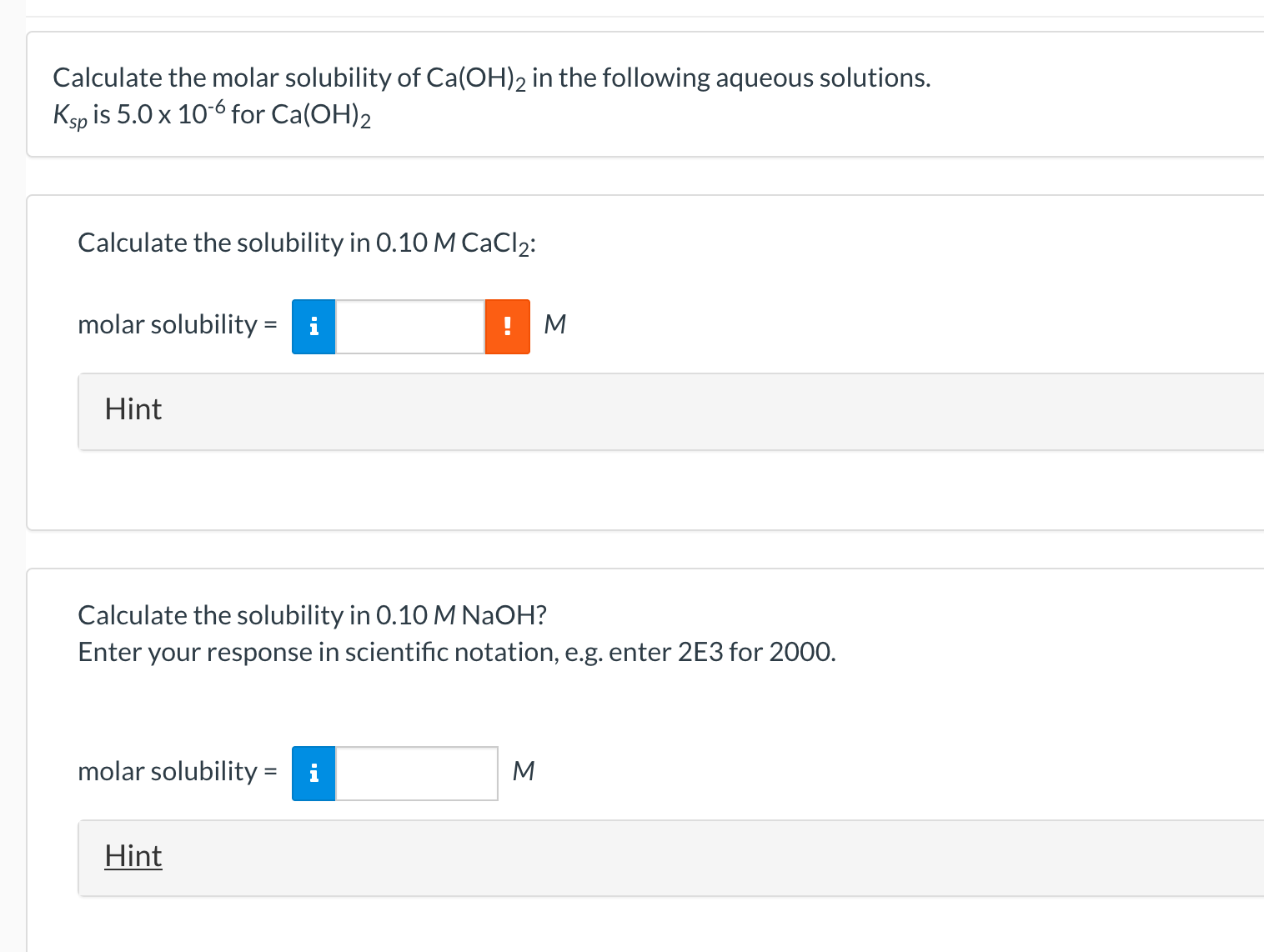
Note that Ksp is only for Ca(OH)2, so only work with Ca(OH)2.
Ca(OH)2 → Ca2+ + 2OH-
a) Ksp=[Ca2+][OH-]2=(0.10+s)(2s)², but s in 0.10+s is deemed negligible.
s=3.54E-3
b) Ksp = [Ca2+][OH-]2=s(0.10)2
s=5.0E-4