The cytoskeleton, molecular motors, and contractility
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
🔹 Cytoskeletal
Q: You look at the cell and discern microfilaments, microtubules, and intermediate filaments. What are they?
A: These are the three main components of the cytoskeleton:
Microfilaments (actin): support cell shape and enable movement.
Microtubules: provide tracks for intracellular transport and form the mitotic spindle.
Intermediate filaments: give mechanical strength and maintain cell integrity.
🔹 Cytoskeletal Polarity
Q: Which cytoskeletal elements have polarity?
A:
Microfilaments (actin) – polarized (+ and – ends)
Microtubules – polarized (+ grows faster, – anchored at centrosome)
Intermediate filaments – non-polar
🔹 Force Exertion by Cells
Q: What is the evidence that cells exert forces on substrates?
A:
Wrinkling of soft substrates beneath migrating cells
Traction force microscopy shows forces at focal adhesions
Deformation of micropillars and elastic gels by cell movement
🔹 Actin Organization: Microvilli vs. Lamellipodia
Q: How is actin organized in microvilli vs. lamellipodia?
A:
Microvilli: tightly packed parallel bundles
Lamellipodia: branched meshwork, highly dynamic
🔹 Actin Branching
Q: How is branching of F-actin achieved?
A: The Arp2/3 complex binds the side of existing filaments and nucleates a new branch at ~70°.
🔹 Actin Polymerization Kinetics
Q: What indicates that the '+' end of F-actin grows faster?
🔹 Actin Polymerization Kinetics
A: In vitro polymerization assays show faster addition of actin monomers at the + end vs. the – end.
🔹 Actin Polymerization Kinetics
Q: How does ATP in actin affect its affinity to the '+' end?
A: ATP-actin has high affinity for the + end, promoting polymerization. After incorporation, ATP is hydrolyzed to ADP, reducing filament stability.
🔹 Actin Polymerization Kinetics
Q: What are the critical concentrations (Cc) of actin at the + and – ends?
A:
+ end Cc ≈ 0.1 µM
– end Cc ≈ 0.6 µM
Calculated by observing the steady-state concentration of free monomers where growth equals loss.
🔹 Formins
Q: What is the role of formins in actin assembly?
A: Formins nucleate and elongate unbranched actin filaments at the + end by remaining attached and promoting addition of ATP-actin.
🔹 Erythrocyte Cytoskeleton
Q: What is the cytoskeletal structure in erythrocytes?
A: A spectrin-actin-ankyrin network forms a flexible mesh network in erythrocytes that maintains biconcave shape and resists shear forces during circulation.
🔹 Myosin Types
Q: What are the three major myosin types in mammals and their functions?
A:
Myosin I: membrane trafficking, endocytosis
Myosin II: muscle contraction, cytokinesis
Myosin V: organelle transport along actin filaments
Be able to draw the structure of the sarcomere.
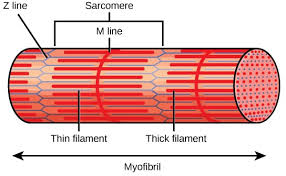
🔹 Sarcomere Structure
Q: How are actin filaments oriented relative to the Z-disk in sarcomeres?
A: Actin filaments anchor at the Z-disk with their + ends toward the Z-disk and extend toward the M-line.
🔹 Sarcomere Structure
Q: What is the role of Titin?
A: Titin acts like a molecular spring, maintaining sarcomere integrity and passive tension during stretching.
🔹 Calcium and Muscle Contraction
Q: How does Ca²⁺ trigger striated muscle contraction?
A:
Ca²⁺ binds troponin C
This causes tropomyosin to shift, exposing myosin-binding sites on actin
Myosin binds and initiates contraction
🔹 Myosin Processivity
Q: What is the relationship between myosin processivity and duty ratio?
A: Duty ratio is the fraction of the ATPase cycle a myosin is bound to actin.
High duty ratio → high processivity (e.g., myosin V)
Low duty ratio → non-processive (e.g., myosin II)
🔹 Myosin in Auditory Hair Cells
Myosin in Auditory Hair Cells
Q: What role does myosin play in auditory hair cells?
A: Myosin I adjusts tension in tip links, modulating the opening of mechanosensitive ion channels during sound detection.
🔹 Myosin in Auditory Hair Cells
Q: What is the role of myosin in auditory hair cells, and how does it relate to tip links and transduction channels?
A: Myosin acts like a tension adjuster in hair cells. It climbs actin filaments in stereocilia to pull on tip links, which control the opening of mechanosensitive ion channels. This maintains proper tension for sound detection and adaptation, resetting the system after stimulation.
🧠 Memory trick: Think of myosin as a “volume knob” that fine-tunes how tightly the “strings” (tip links) are pulled to open the “gates” (channels).
🔹 Microtubule Structure
Q: Describe microtubule organization.
A:
Built from α- and β-tubulin dimers
+ end grows/shrinks; – end is anchored
Made of 13 protofilaments forming a hollow cylinder
Contains a seam where protofilament alignment differs
Exists as singlets (cytoplasm), doublets (cilia), or triplets (centrioles)
🔹 Motor Proteins & Directionality
Q: What are the directions and speeds of myosin, kinesin, and dynein?
A:
Myosin: moves toward + end of actin (~0.5–3 µm/s)
Kinesin: moves toward + end of MTs (~0.8 µm/s)
Dynein: moves toward – end of MTs (varies; ~1–2 µm/s)
🔹 Kinesin Family Functions
Q: What are the functions of kinesins 1, 5, and 13?
A:
Kinesin-1: vesicle/organelle transport
Kinesin-5: pushes spindles apart during mitosis
Kinesin-13: depolymerizes microtubules at ends (catastrophe factor)
🔹 Diffusion Distances
Q: How is diffusion distance (σ) related to time in different dimensions?
The diffusion distance is measured as a root-mean-square deviation from the origin ()
A:
1D: σ = √(2Dt)
2D: σ = √(4Dt)
3D: σ = √(6Dt)
Where D is the diffusion coefficient and t is time.
🔹 Diffusion Coefficient Factors
Q: What physical factors affect the diffusion coefficient (D)?
A:
Temperature (↑ T → ↑ D)
Viscosity of the medium (↑ viscosity → ↓ D)
Particle size (larger → slower diffusion)
According to the Stokes-Einstein equation