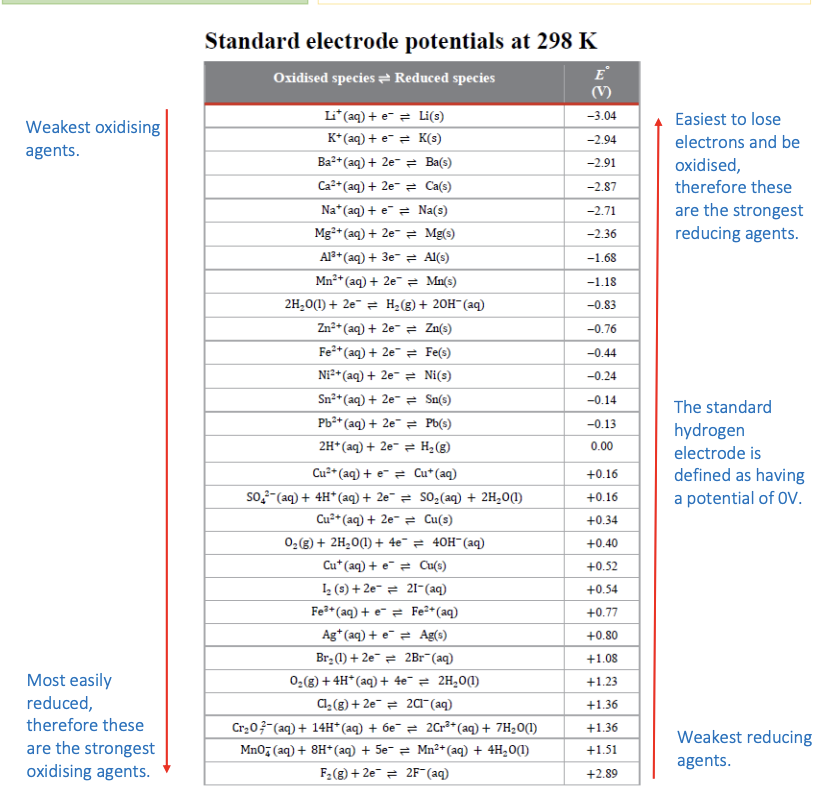
Standard electrode potentials
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
5 Terms
E°cell value from strongest to weakest oxidising agents of K, Cl2, Al and I2
strongest = most easily reduced i.e gains electrons
Cl2(+1.68V), I2(+0.54V), Al, K
strongest to weakest: Ba(-2.94V), Ni(-1.68V), Ag, F2
How to calculate E°cell:
E°cell = E°red - E°ox
if positive = reaction is spontaneous
if negative = reaction is not spontaneous
*A voltage greater than the E°cell would need to be applied to get the reaction to occur
calculate E°cell of Zn and I2:

Describe the purpose of the standard electrode potential table.
The table provides a comparison of the ease for reduction to occur by giving half-cell potentials and allows us to work out which species is reduced and which is oxidised for a spontaneous reaction to occur.
Annotate the table below with key features: