Parkinson's and 3D structures
1/41
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
42 Terms
Cause of PD
Loss of dopamine producing neurons in substantia nigra/midbrain
Symptoms of PD
Tremor, difficulty with movement
Base treatment of PD
Dopamine precursor supplementation (L-DOPA)
Why L-DOPA and not dopamine?
Dopamine doesn’t cross the barrier
Does L-DOPA treat DP?
It only alleviates the symptoms
Clues to understanding molecular mechanisms
Pathogenicity and genetics
Pathogenicity
Hallmarks (pathways) of neurons that make them vulnerable
Genetics
Look into genetic mutations
Look into the functions of the mutated genes
Pathway through pathogenicity
PD and mitochondrial damage
Story behind that pathway
MPTP in MPPP (recreational drug)
What is MPTP?
Inhibitor of mitochondrial respiration/of one of the proteins in the chain (Complex 1)
Result of MPTP poisoning
Loss of SNc DA neurons bc of mitochondrial inhibition
Why did SNc DA neurons die?
Bc of their high metabolism (high dependency on mitochondria)
What other aspect of mitochondria affects neurons?
Mitochondrial respiration creates oxidative stress = damaged mtDNA and proteins
Pathway through genetics
Over 40 genes associated
Monogenic form of PD (5-20%)
Only one gene mutation is sufficient to cause the disease
Most important onset factor
Age
Early-onset cases
Under 50 yo
Late-onset
Over 60 yo
Are the targeted genes only expressed in neurons?
No: everywhere in the body
Which gene mutations cause early-onset recessive (both alleles) PD?
Parkin (120m) and PINK1 (200m)
Mutations in Parkin and PINK1 cause…
1% of all PD cases
More than 60% of early-onset cases
How can mitochondria be damaged?
Mitochondrial depolarization by CCCP or respiration inhibitation by MPTP/rotedone
Result of damage
Recruitment of Parkin by mitochondria
Class of PINK1
Enzyme/Protein kinase that phosphorylates ubiquitin in damaged mitochondria
How is Parkin recruited to mitochondria?
P-ubiquitin recruits it and activates it
Activity of Parkin
Adds ubiquitin to mitochindrial proteins = damaged mitochondria
Approach to test if PINK1-Parkin mt quality control is defective
Structural biology for mechanistic insights
How do we get 3D atomic model of a protein?
X-ray crystallography
X-ray crystallography
Protein crystals that are frozen in a loop and exposed to high-intensity X-rays = electron density map from the diffraction pattern
Requirements of X-Ray crystallography
High content of purified proteins
Proteins conditions that yield crystals
Key element of crystals
The protein copies must all have the same orientation in the crystal
AI and 3D atomic models
Ai can predict it but not 100% accurate
Advantages of X-Ray crystallography (2)
Domain organization
Catalytic site localization (RING2-RING0 domains interface)
Where does the ubiquitin from Parkin come from?
Catalysed transfer from an E2 enzyme
Intermediate of the transfer
Thioester on RING2 (linked to the earliest case of PD (18 yo))
How is Parkin auto-inhibited?
Distance between E2-site (RING1) and catalytic site (RING2) is too long (50 Å)
REP blocks access to E2-site (gatekeeper)
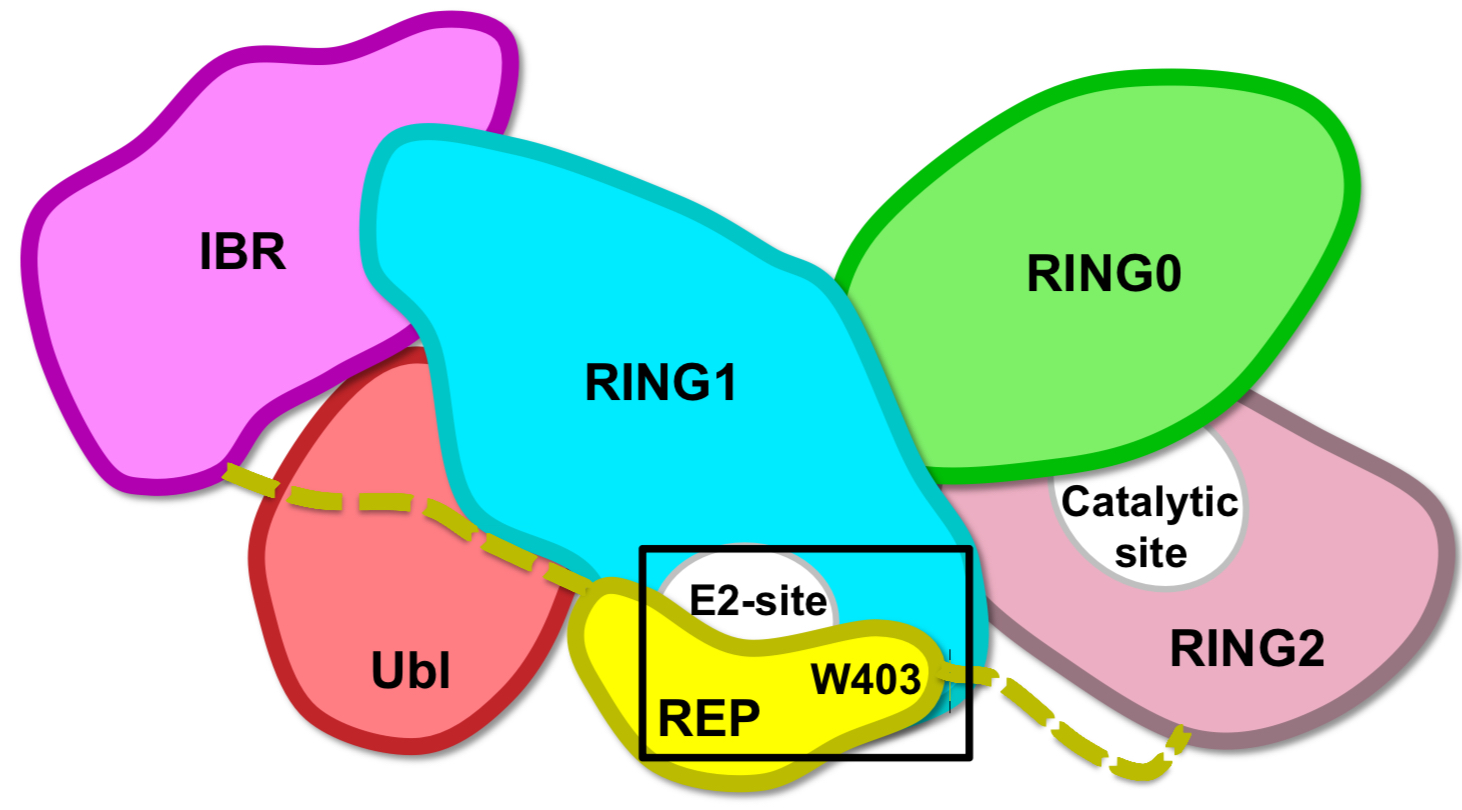
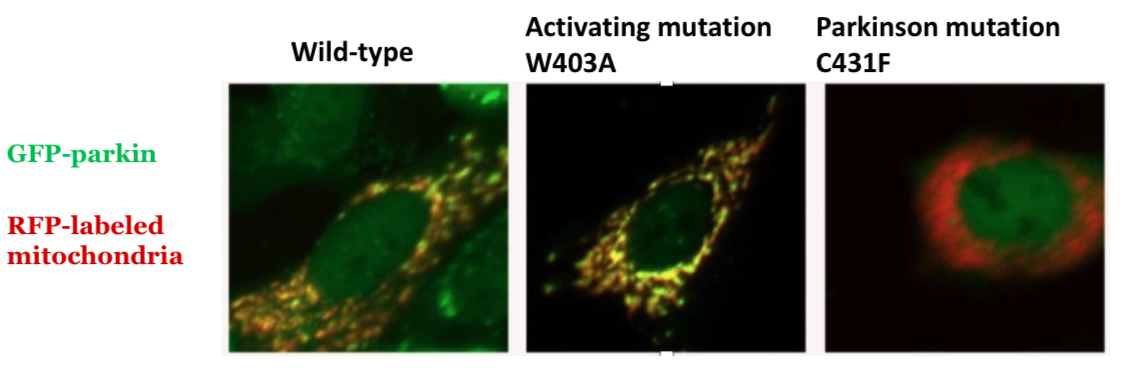
Hypothesis to release auto-inhibition
Mutation of chain residue that anchors REP: Trp403 into Ala/W403A = accelerated recruitment
Role of Lys161/211
These residues are +ve, bind the phosphate and are mutated in PD = therefore needed for Parkin activation through phosphorylation
PD mutations that can be rescued by activating W403A mutation
Missense mutations: K161N and K221N
Activating mutations that rescue
W403A and F146A
PD mutations that cannot be rescued
Active site mutations (C431F) and E2-binding site mutations (T240R)