11CHEM: Unique properties of water and Water Treatment
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
36 Terms
Describe the structure of a water molecule
. made up of two hydrogen atoms bonded to an oxygen atom
Shape and polarity of water
. lone pairs cause bent shape
. uneven distribution of electrons = polarity
. concentration of charges —> very strong dipoles —> very strong hydrogen bonding
—> High MP/BP: large amounts of energy needed to break bonds
—> dissolves ionic substances: strong positive and negative poles allows for strong attraction to anions and cations
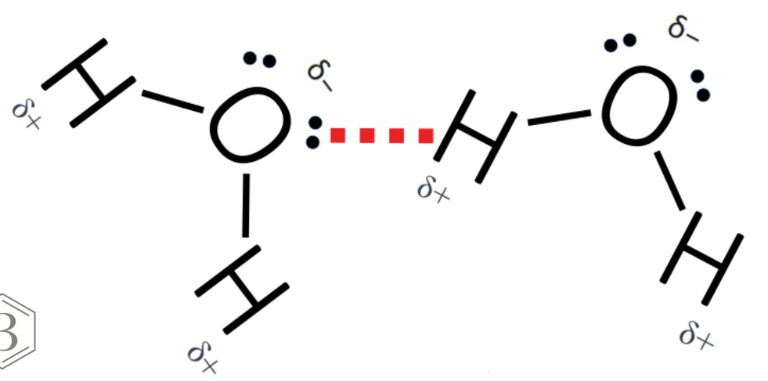
Describe how and why water molecules bond together
. Each end of a water molecule has a slight electric charge (so is polar)
--> uneven distribution of charges across a molecule making one end positive (H) and the other negative (O) = polarity
--> The positive hydrogen ends of one water molecule attract the negative oxygen ends of nearby water molecules causing them to stick together like weak magnets
--> This attraction causes water molecules to form temporary bonds that break easily = hydrogen bonds
What is the general reason for water’s unique properties
. Many of water's unusual properties occur because of the attraction among its polar molecules
List the unique properties of water (6)
Cohesion
Adhesion
Capillary action
Surface tension
The ability to dissolve many substances
High specific heat
Unique property of water: Cohesion
. The tendency for water molecules to form weak bonds and stick to each other
. Because of cohesion, water molecules remain joined together as they move within or between the cells of organisms
Unique property of water: Adhesion
. the tendency of water to stick to other substances
.EG: when you add water to a graduated cylinder
--> At the surface, water creeps up slightly at the sides of the cylinder, forming a curved surface (meniscus)
. allows water to stick to the sides of blood vessels or to the vascular tubes in plants
Unique property of water: Capillary action
. is the combined force of attraction among water molecules and with the molecules of surrounding materials causing a liquid to climb upward against the force of gravity
. is the result of both adhesion to the sides of a surrounding material and cohesion of the water molecules to each other
. It allows water to move through materials with pores inside
. It causes water molecules to cling to the fibers of material like paper and cloth
. Capillary action along particular cloth fibers pulls water away from your skin keeping you dry
. EG: Both adhesion and cohesion allow water to move in one continuous column from a plant's roots to its leaves (upward movement = capillary action)
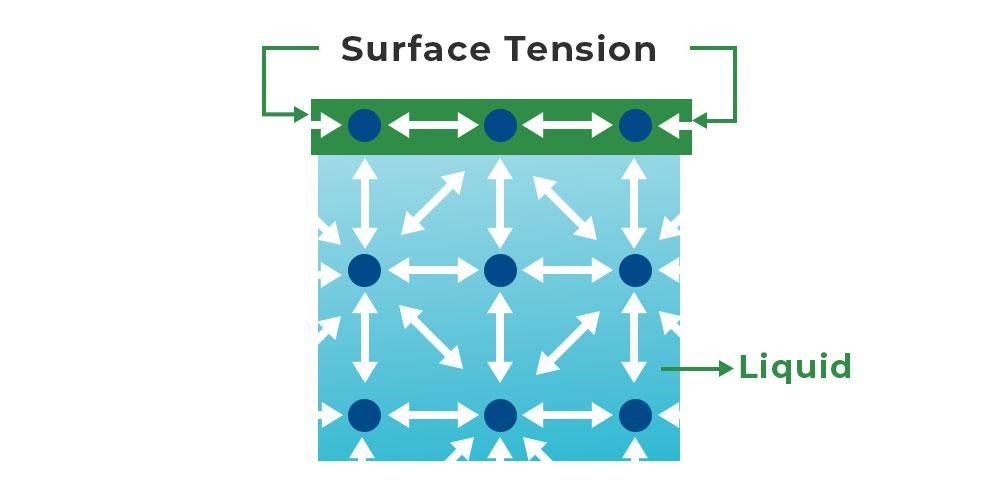
Unique property of water: Surface tension
. Is a special example of cohesion
. Is a force that acts on the particles at the surface of a liquid
. Is the tightness across the surface of water that is caused by polar molecules pulling on each other
. In liquid water, each water molecule is pulled in all directions by other water molecules
. At the surface of the water, however, the attractive force of other water molecules pulls only downward and sideways
--> This force causes molecules at the surface to be held more tightly together, forming a kind of skin at the water's surface
. Small insects, such as water striders, can walk on water by taking advantage of this surface tension
. surface tension = the amount of energy required to stretch or increase the surface of a liquid by a unit area
. a molecule within a liquid is pulled in all directions, whereas a molecule on the surface is only pulled to the interior
—> as a result, there’s a tendency for the surface area of the liquid to be minimised
Special properties of water: Universal solvent (solubility of substances in water)
. water can dissolve more substances than any other known substance, mainly due to its polarity
. ionic substances soluble in water (as the charged ends of the water molecule attract the molecules of other polar substances): positive + negative ions of the ionic compound are attracted to the oppositely charged ends of the water molecule, causing the ions to separate from each other and disperse throughout the water
—> can dissolve: sugar, bleach, salt, CO2, O2
—> EG of dissolving salt: the negative side of water (O) forms bonds with the Na+ and the positive side (H) is attracted to Cl- ions in a crystal of salt (NaCl), so as it pulls these ions into solution, the crystal dissolves
—> The ability of water to dissolve many substances allows water to deliver essential nutrients to cells in plants, animals, and other organisms (water dissolves nutrients in our food)
. Non-polar substances (dispersion forces only) are insoluble: CANNOT disrupt hydrogen bonds between water molecules (non-polar molecules tend to clump together within the water)
—> are hydrophobic
—> EG: oil and wax
Unique property of water: High specific heat
. the amount of heat needed to increase the temperature of 1 kg of a substance by 1°C
--> The unit of specific heat is joule per kilogram per degree Celsius (J/kg • 0G)
. Compared to other substances, water requires a lot of heat to increase its temperature
. The specific heat of water is very high (4,184 J/kg • °C)
. Therefore, water takes a long time to heat up or cool down
. Water has a high specific heat because of the strong attraction among water molecules (cohesion)
. allows lakes, streams, and ocean ecosystems to maintain stable temperatures, even if air temperatures change dramatically
--> air over water is cooler than the air over land on hot days
. The high specific heat of water also helps your body to maintain a constant internal environment
Density of water in liquid and solid phase
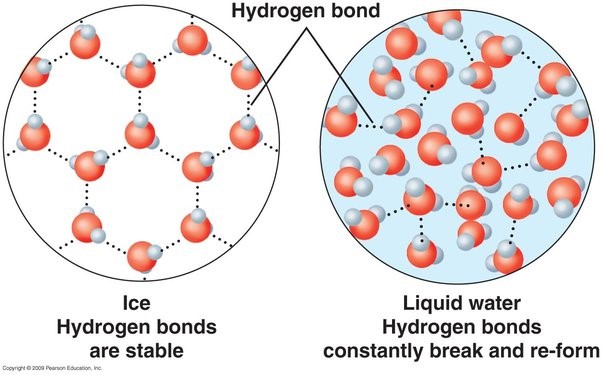
. as water freezes, its density decreases = ice floats
—> as the water crystalises, hydrogen bonding allows the water molecules to form a more open, crystalline structure (hexagonal arrangement = density decreases)
. results in a larger volume for the same mass
. When water freezes, its molecules lose energy and get stuck in a lattice structure in which they are farther apart from each other than in their liquid state, thus making ice less dense than water
Water molecule structure diagram
Solution, solvent and solute
. solute is the substance that dissolves
. solvent is the substance that does the dissolving
. solution is the resulting homogenous mixture (mixture that forms when 1 substance dissolves another)
Describe the homogenous property of ALL solutions
. solutions are the same throughout
. the solvent and solute cannot be distinguished from each other
. dissolved particles are too small to see, so solutions are clear but may have a colour
Dissolution
. a chemical process where a solute dissolves into a solvent to form a homogeneous mixture (solution)
—> when both solute + solvent are liquids they are said to be miscible (forming a homogenous mixture when added together)
What does dissolution require
. for a substance to dissolve, the attractive forces between solute + solvent particles must be able to overcome the forces between the solvent particles and between the solute particles
. solute-solvent interactions ≥ solute-solute interactions, solvent-solvent interactions
What substances will polar and non solar solvents dissolve
. polar solvents: dissolve polar solutes + charged ions
. non-polar solvents: dissolve non polar molecules (eg: oil (non polar) doesn’t dissolve in water (polar))
Water and dissolving molecular compounds
. some small molecules can dissolve in water
—> eg: ammonia (NH3), hydrogen chloride (HCl)
—> they can do this if they contain a polar functional group (eg: OH) which allows them to form hydrogen bonds with water, OR if the molecule splits into ions (ionises)
—> eg: ammonia can dissolve in water by forming hydrogen bonds with water or by reacting to form the ammonium ion (NH4+)
Dissociation
. the chemical process where a compound breaks apart into simpler constituents, such as ions
—> thus is the process of dissolving a solid ionic compound
Water and dissolving ionic compounds
. for ionic compounds the positive ends of the water molecules are attracted to the negatively charged ions and vice versa
—> ion-dipole interactions between negative ions and H atom, and positive ions and O atom
Dissociation example equation
NaCl (s) —>H2O (l)—> Na+ (aq) + Cl- (aq)
Soluble ions (snape)
sodium (Na+)
nitrate (NO3-)
ammonia (NH3)
potassium (K+)
ethanoate (CH3COO-)
Potable water
. water safe to drink
. drinking water that meets regulatory quality standards to protect human health, ensuring it is free from harmful chemical pollutants and waterborne diseases
What does potable water contain
. low levels of: dissolved salts, microbes
. most contains some dissolved salts
Where is potable water obtained from
Freshwater supplies:
. rainwater dissolves some gases from air as it falls to ground
—> this freshwater collects:
. underground (groundwater)
. in lakes
. in rivers
Desalination of salty water
Treatment of sewage/agricultural water
How is potable water from these freshwater sources produced
Choosing an appropriate source of freshwater
Filtration: Passing the water through filter beds (sand+gravel layers) to remove insoluble solid particles
Sterilisation: Sterilising (killing micro organisms by chlorine, ozone, or passing UV light through)
. filtration + sterilisation
. requires sterilising agents + filtration equipment
. easiest method: least equipment/energy/cost
Sterilising agents
. substances or methods used to destroy or deactivate all forms of microbial life
. chlorine, ozone, UV light = used to sterilise freshwater
What is required if freshwater supplies are limited
. may have to desalinate (remove salt) salty water or sea water
Desalination method evaluation
. distillation or using membranes
. both require lots of energy
. hardest method: requires most energy
Describe the 2 main ways in which desalination occurs
Distillation:
. separates mixtures with different boiling points
. separates the salt from the pure water
. sea water boiled
. water molecules boil (impurities stay behind)
. steam is cooled + condensed to pure water
. high energy from heat
Using membranes:
. reverse osmosis
. uses pressure to force saltwater through a semi-permeable membrane, separating the water molecules from dissolved salts + other impurities (as dissolved substances cannot pass through)
. water moves from an area of high salt concentration to low
. high energy from pressure
Disadvantage of desalination methods (energy)
. both methods require lots of energy
. distillation = energy needed to boil water
. using membranes = energy needed to pressurise water
THUS: lots of energy = expensive so desalination is rarely used
Describe causes of waste water production and its treatment requirements
. urban lifestyles + industrial processes produce large amounts of waste water
. requires treatment before being released into environment
. sewage + agricultural waste water requires the removal of: organic matter + harmful microbes
. Industrial waste water requires removal of organic matter + harmful chemicals
Treatment of waste water method evaluation
. screening, sedimentation, digestion (aerobic + anaerobic)
. several steps, requires large treatment plant
. moderate method: requires more equipment but less energy than desalination
Describe the 4 steps to sewage water treatment (pg 40 diagram)
Screening + grit removal:
. screening removes large solid particles (grit) by passing the sewage through a screen
Sedimentation (to produce sewage sludge + effluent):
. sedimentation allows the small solid particles (sediment) to sink to the bottom of the tank forming sewage sludge while the liquid (effluent) remains above
Anaerobic digestion of sewage sludge:
. the sewage sludge is dried + anaerobically digested (broken down by micro organisms in absence of oxygen)
. the digestion of sewage sludge removes organic matter
. produces methane which can be burned to produce biogas used to make electricity
. dried sludge can be used as fertiliser
Aerobic biological treatment of effluent:
. the effluent is aerobically digested (broken down by micro organisms in the presence of oxygen) —> removes organic matter + harmful microbes
General 6 Steps of water treatment
Coagulation: chemicals are added to make the particles of insoluble solids stick together
Flocculation: agitation is used to make the small clumps stick together to form large clumps
Sedimentation: large clumps sink to the bottom of a tank and are separated from the water
Filtration: the water is passed through sand and/or gravel filters to remove any remaining solids
Disinfection: chlorine, ozone or UV are used to kill any microbes
Fluoridation: fluoride is added to protect teeth from decay