FUTURE PROSPECTS FOR CELL & MOLECULAR THERAPY
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
45 Terms
what is cell-based therapy
the administration of cells as ‘living agents’ in patients to fight disease
what are challenges of cell therapy
identification of the appropriate cell source
generation of a sufficiently viable, potent and safe product
development of scalable manufacturing processes
from what sources can cells be derived from in cell based therapy
autologous cells
allogeneic cells
xenogeneic cells
outline autologous cells
patient’s own cells
bone marrow-derived haematopoietic stem cells (HSCs)
immune effector cells isolated from peripheral blood
induced pluripotent stem cells (iPSCs)
what are advantages and disadvantages of autologous cells
advantages
avoid immune response (good long-term engraftment)
iPSCs can turn cells that have terminally differentiated into pluripotent cells
disadvantages
dependence of product quality upon the patient’s health
high manufacturing costs
outline allogeneic cells
human origin but from an individual distinct from the patient
cells usually encapsulated in biopolymer matrices to prevent immune recognition
what are advantages and disadvantages of allogeneic cells
advantages
potentially scalable production
source will be a healthy human being so cells will be of high quality
theoretically unlimited supply
what are examples of allogeneic cells
natural killer cells (NK)
mesenchymal stem cells (MSCs)
outline xenogeneic cells
of non-human origin
often porcine cells
what are challenges of using xenogeneic cells in cell-based therapy
overcoming host immune rejection
patients need immunosuppressants for the rest of their life
often they are very sick already so giving them immunosuppressants weakens their immune system even further so they are more likely to get sick again
some patients may not be able to receive porcine cells due to religious/ lifestyle
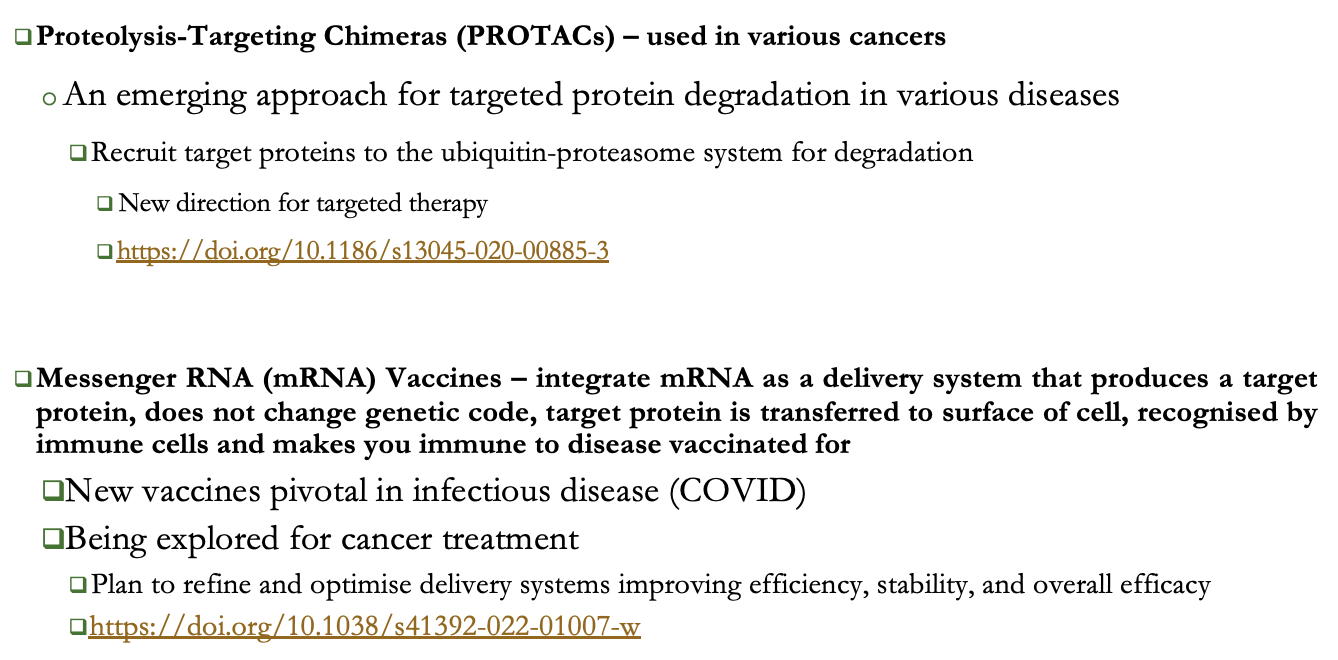
image showing challenges at each stage of the cell therapy production process
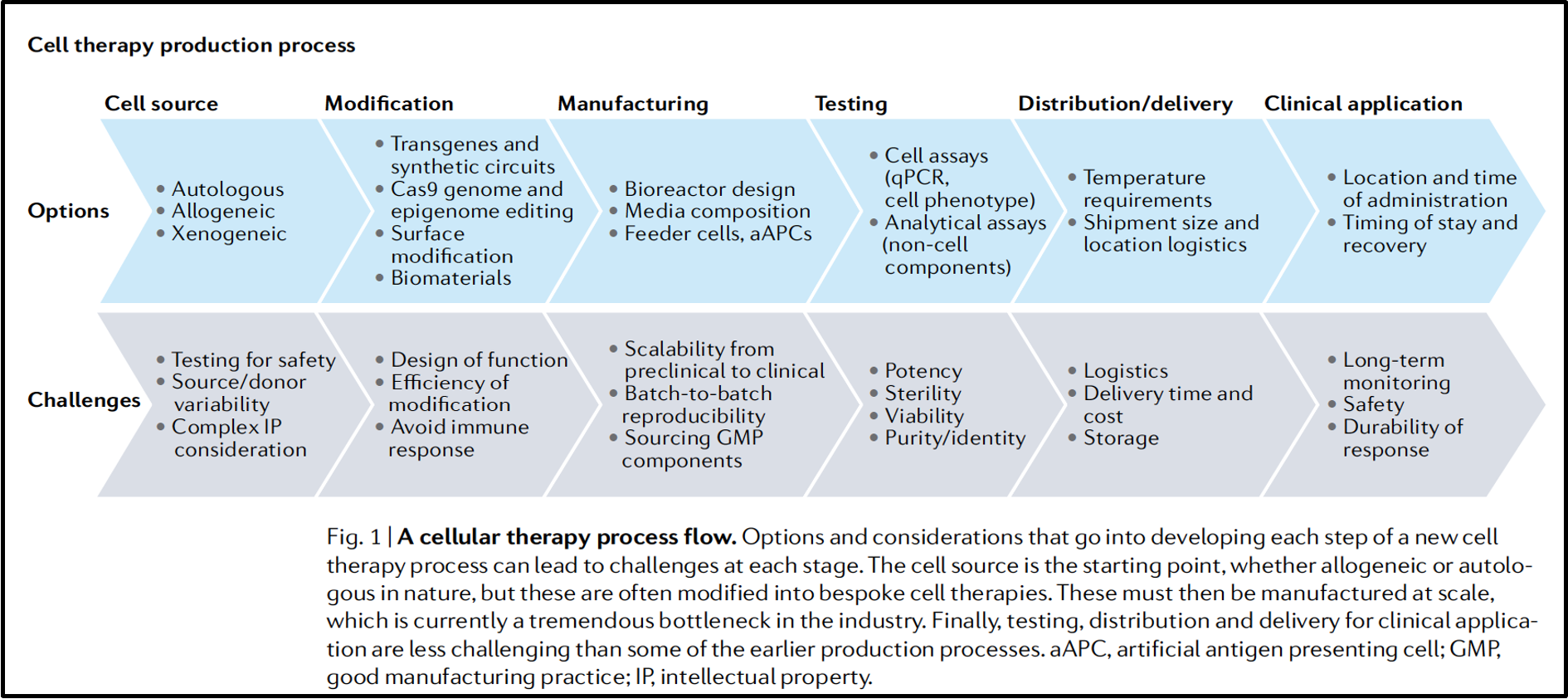
table showing dental application of cell-based therapies

outline Gintuit
made of isolated living cells from human skin cells (keratinocytes) and grown with bovine collagen
cross-talk between cell release growth factors and cytokines that promote tissue regeneration
replace palatal grafts used for receding gums
available in US not UK
what advantage does Gintuit have over the traditional gold standard graft method
Gintuit is much better tolerated by the patient
traditionally, tissue was grafted from the palate but this is very painful
outline CAR T-cell therapy
precision medicine: type of cancer immunotherapy
T-cells are re-engineered to produce chimeric antigen receptors (CAR) that recognise antigens of malignant cells
‘giving patients a living drug’
what are the risks of CAR T-cell therapy
cytokine release syndrome (CRS) due to large immune response because T-cells release cytokines
patients feel severe fatigue, fever and breathing difficulties
for CAR T-cell therapy to work, it must be known what cancer the patient has, why is this?
patient’s own T-cells are isolated
T-cells are modified to recognise the specific antigens produced by tumor cells
therefore must know what cancer the patient has
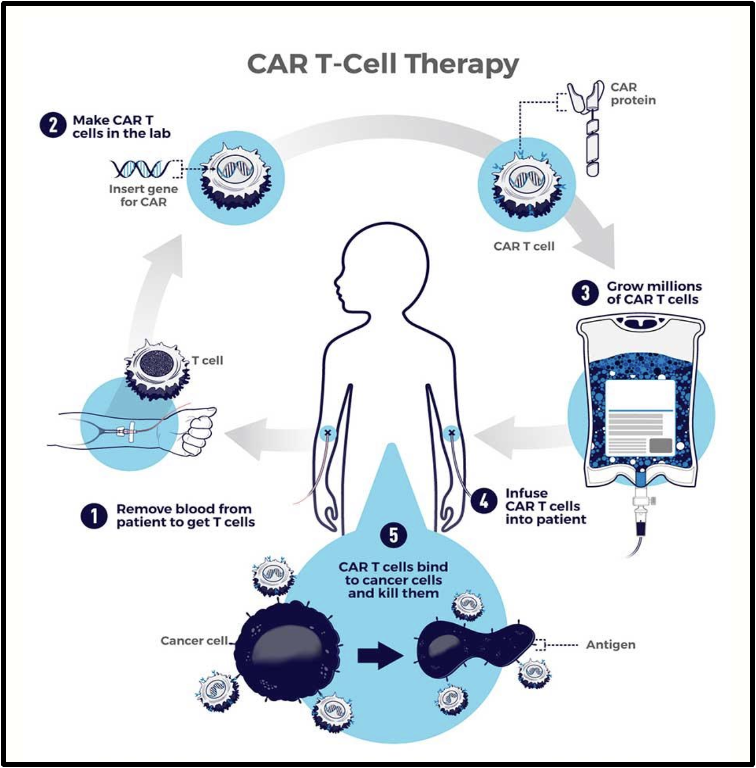
list of FDA approved CAR T-cell therapies
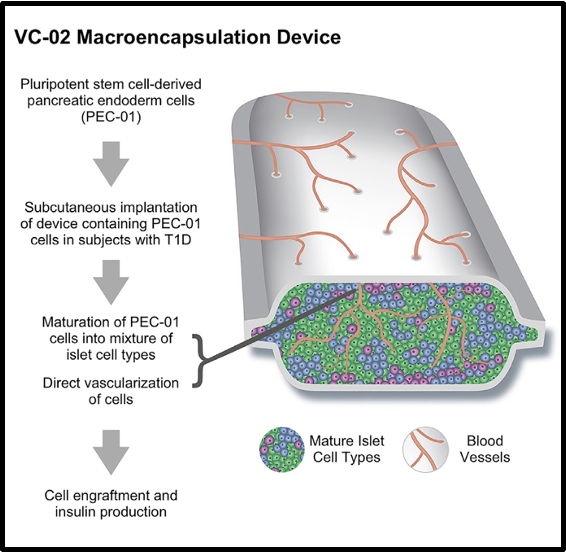
give an example of autologous based therapies
stem cell derived insulin-producing cells can theoretically be generated in endless quantities » large amount of insulin generated
ongoing clinical trials of stem cell based therapies to cure type 1 diabetes
transplants pluripotent stem cell derived pancreatic endoderm cells in macroencapsulation devices which can be vascularised to receive signals from the human body
—
CT do not prove therapeutic levels of insulin secretion or provide unequivocal evidence of clinical benefit
therapeutic levels of insulin produced was not enough when tested in human patients
what can cell therapy be theoretically used for in dentistry
use of dental stem cells for tooth regeneration
biomaterials/ scaffolds for regenerative dentistry (maxillofacial incl. soft tissues)
bioengineered tooth erupted and physiologically similar to natural teeth
autologous transplantation of bioengineered tooth germ reconstructed using a patient’s own stem cells


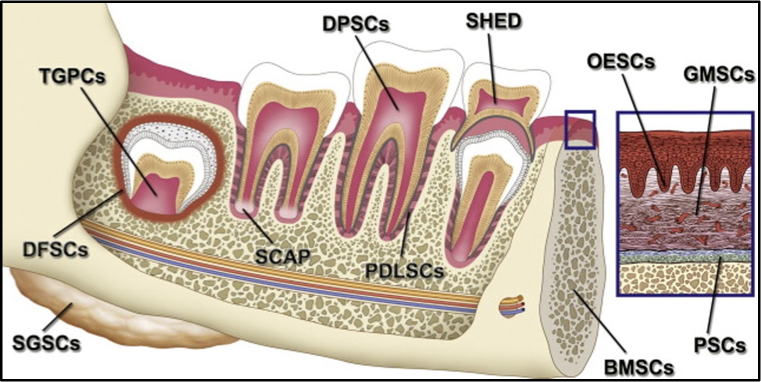

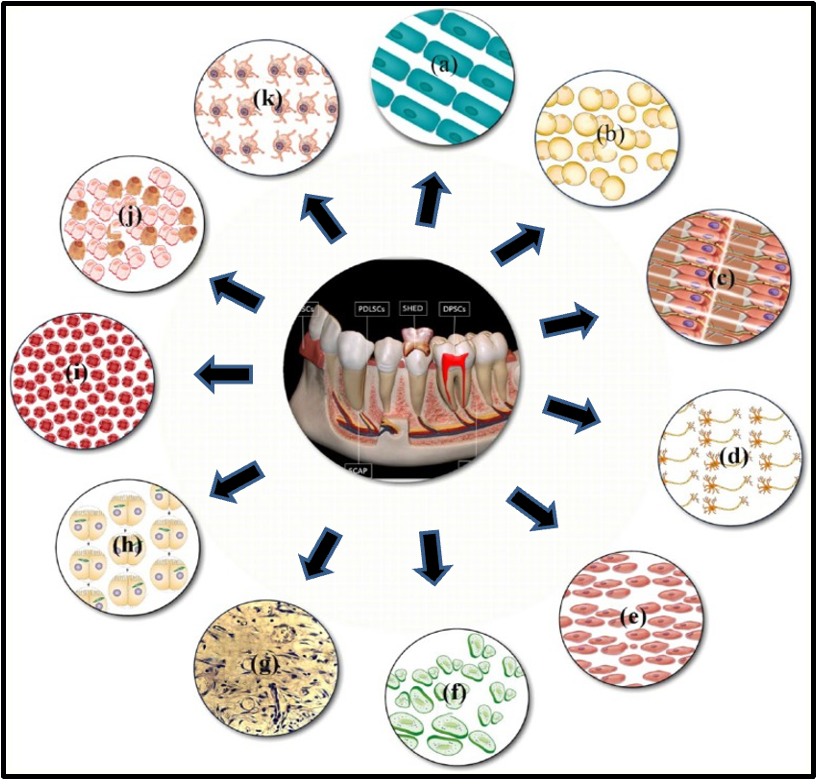
what cell types can be derived from dental stem cells
a) cementoblast
b) adipocyte
c) odontoblast
d) neuronal cells
e) myoblast
f) chondrocyte
g) pulp cells
h) hepatocyte
i) endothelial cell
j) osteoblast
k) melanocyte
give an example of a clinical application of stem cell therapy
use of deciduous dental pulp stem cell (DDPSC) for unilateral cleft defects
outcomes: significant results of bone regeneration compared with traditional methods of bone grafting
Tanikawa DYS et al. (2020)
what other condition can stem cell therapy be used to treat
apexification (procedure that closes the tip of an open apex of an immature tooth with a non-vital pulp)

what are the main challenges of cell therapy in dentistry
achieving the correct type, size, shape and colour of the original tooth
achieving full function of the regenerated tooth with vascularisation, innervation and binding to supporting tissues
what is tolerance induction
tolerance induction: giving the patient a little at a time
how can cell therapies be modified
using biomaterials
using gene editing
outline biomaterials in cell therapy modifications
improves the delivery, viability, retention and safety of therapeutic cells
encapsulation is effective in preventing immediate rejection of allogeneic cells
long-term effect is a challenge due to foreign body response
how can graft cell survival be improved
changes in physical properties
size
shape
surface
morphology
roughness
topography
geometry
outline gene editing in cell therapy modifications
cell-based therapies will almost certainly progress with the aid of genome and epigenome editing tools i.e. gene therapy
engineering approaches:
genetic and/ or epigenetic modification
CRISPR and CRISPR-associated (Cas) proteins
double strand breaks (DSBs)
what are the concerns for engineered cells in gene editing (cell therapy modifications)
DSBs can cause harmful genomic rearrangements
CRISPR-Cas-based based editors (without creating a DSB)
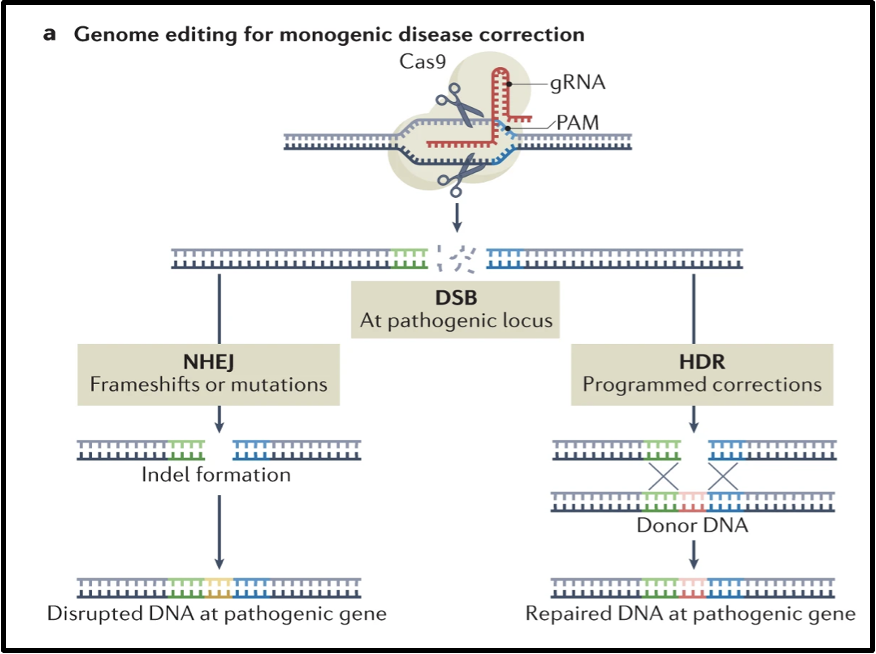
what is gRNA
gRNA = guide RNA
with Cas 9 so Cas 9 knows where to cut the genome
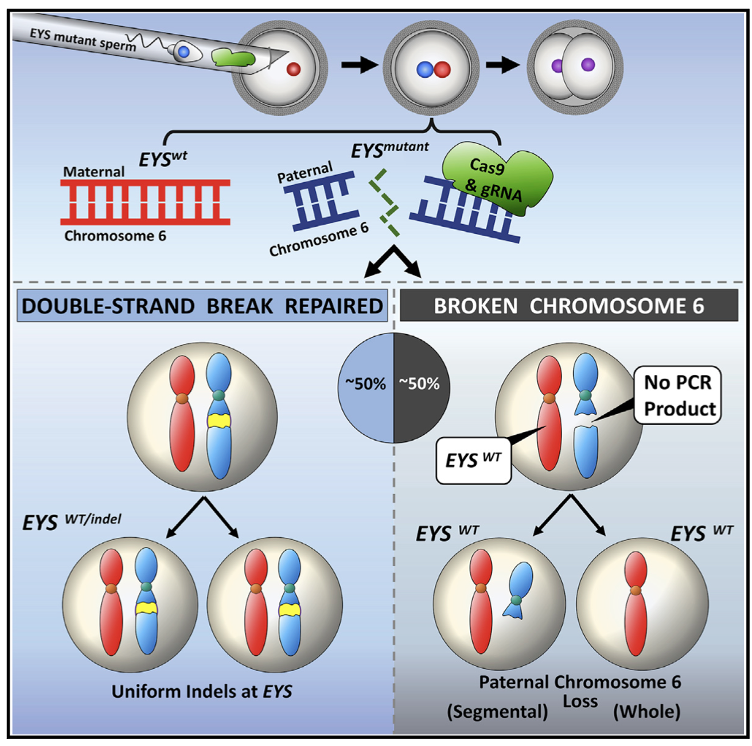
what are problems with CRISPR-Cas9
CRISPR-Cas9 gene editing in early human embryos leads to frequent loss of the targeted chromosome
this indicated that human germline gene editing would pose a substantial risk for aneuploidy (abnormal number of chromosomes in a cell)
therefore cannot be used in vivo safely
what is a major issue with CRISPR technology
deliberately producing double stranded breaks means repairs can go wrong
there are also lots of repetitive areas of DNA so if gRNA is produced incorrectly, there can be a lot of double stranded breaks in DNA
what is gene therapy
a technique for correcting defective genes responsible for disease development e.g. replace a missing/ mutated gene, add genes to fight the disease, turn off genes causing diseases
vectors deliver genes to patient’s target cells
vectors are commonly viruses (retro, adeno or adeno associated)
introduction of a foreign piece of DNA into genome
what does gene therapy success depend on
good molecular strategy and a safe, efficient and specific gene delivery system
viral vectors - highest efficiencies but are associated with immunogenicity
non-viral vectors - safer but not as efficient
outline ex vivo gene therapy
tissue cells from patient are cultured and transduced with modified vector (cells are treated with virus)
transduced cells that produce therapeutic protein are infused back into the body’s genome (transfection)
outline in vivo gene therapy
the modified vector is directly injected into the body
the therapeutic gene is inserted into the virus, virus is injected directly into the body
outline gendicine
first commercially available gene therapy in 2003 approved by China FDA
recombinant human p53 adenovirus - injected directly into tumour and induces tumour cell apoptosis, senescence or autophagy
used mainly for head and neck squamous cell carcinoma (intra-tumoral injection)
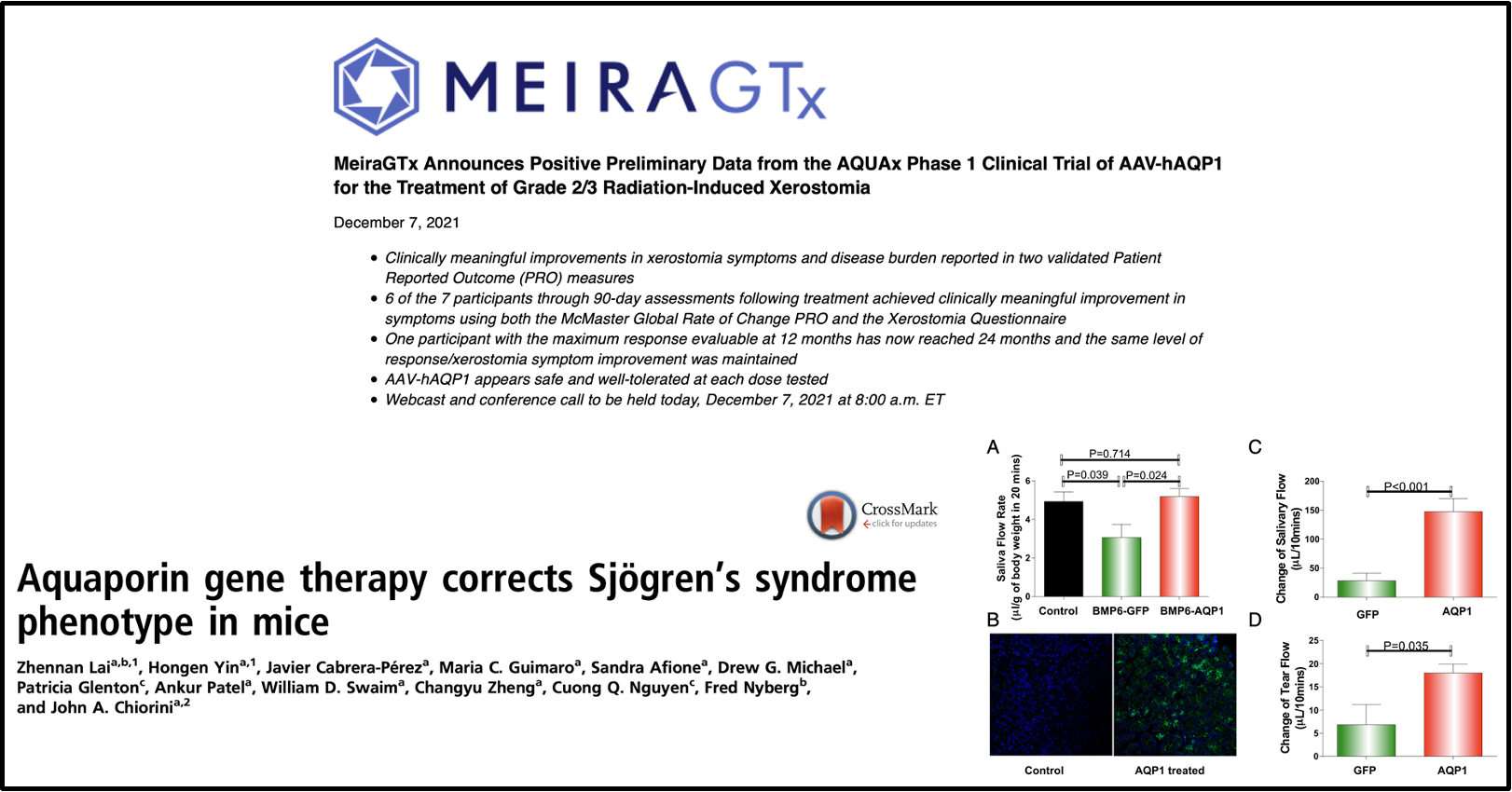
recent example of in vivo gene therapy
adds back aquaporin » improved saliva flow rate and tear flow
what are challenges in gene therapy
toxicity, immune and inflammatory responses of viral vectors
DNA integration into the genome
many human diseases are multigene disorders so gene therapies that target multiple genes need to be developed
seem effective in monogeneic conditions
ethical aspect
outline the ethical challenges in gene therapy
how can ‘good’ and ‘bad’ uses of these technologies be distinguished?
who decides which traits are normal and which constitute a disability or disorder?
will the high costs of gene therapy make it available only to the wealthy?
could the widespread use of gene therapy make society less accepting of people who are different?
should people be allowed to use gene therapy to enhance basic human traits such as height, intelligence or athletic ability?
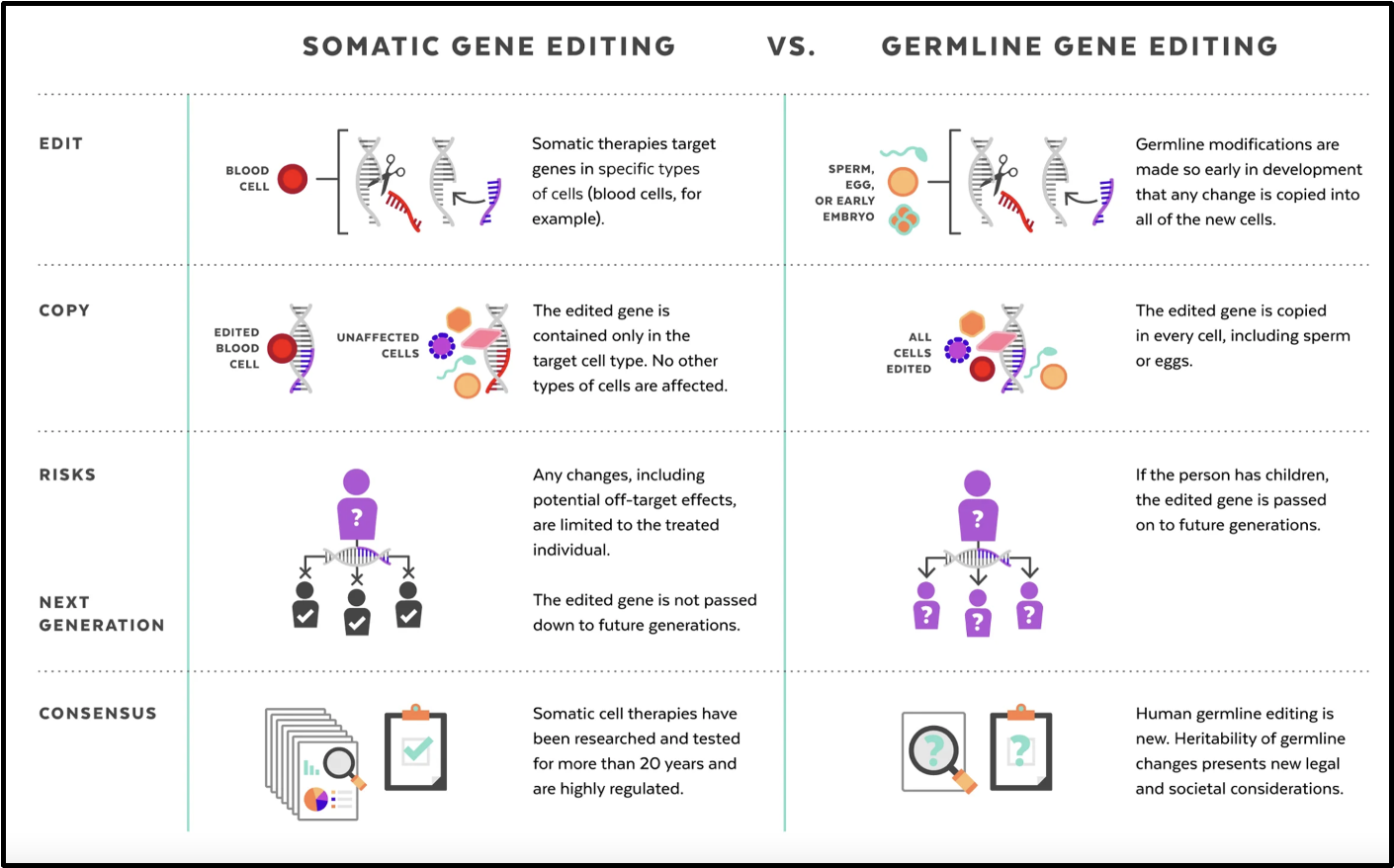
state the differences between somatic VS germline gene editing
other molecular therapies