Lecture 6: enzyme regulation and inhibtion
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
44 Terms
Define enzyme inhibitors
Molecules interfering with enzyme activity, slowing/ halting catalysis
What is the hierarchy of inhibition
Specific → Reversible → Competitive, Non-competitive and Uncompetitive
Specific → Irreversible
Non-Specific
What is specific inhibition
Only one type of enzyme inhibited
Reversible reaction meaning
The EI binding complex is in equilibrium
And non-specific
Inhibits al enzymes, doesn’t discriminate
What is KI
The inhibitor constant - indicates how potent an inhibitor is (how much EI is formed)
Does a competitive inhibitor permanently inactive the enzyme
No
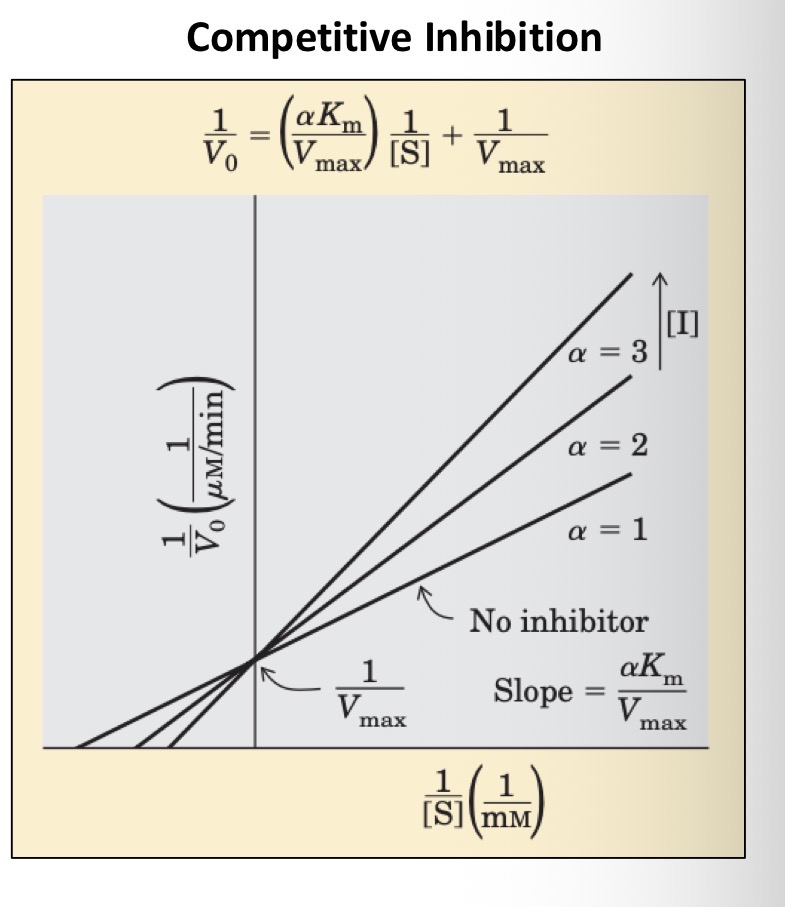
What is the action of a competitive inhibitor
Competes with substrate for the active site, only I or S can be bound at one time
Write out Michaelis-Menten
V0 = Vmax [S] / aKm + [S]
What does a mean (equation)
a = 1 + [I]/KI
What does aKm mean
The observed Km in the presence of an inhibitor (called the apparent Km)
What happens if [S] » [I]
The reaction will be unaffected and will tend towards original Vmax as it is more likely that the substrate will bind than the inhibitor
What happens to Km in the presence of a competitive inhibitor
Increases
What happens to Vmax
Unchanged
Why is ethanol used as a competitive inhibitor for alcohol dehydrogenase
It binds instead of methanol, so formaldehyde (very toxic) isn’t formed as they have the same functional group (CH2OH) so both fit into the active site
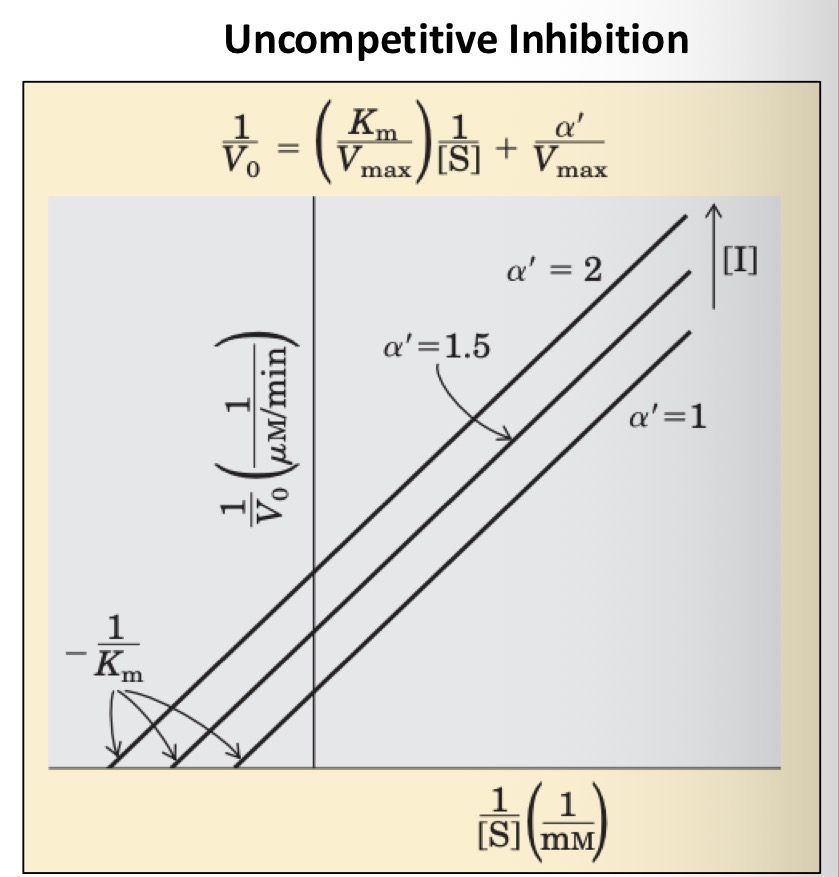
Where does an uncompetitive inhibitor bind
A site distinct from the substrate active site and binds only to the ES complex
Can the substrate in an ESI complex be converted to product
No
What does KI’ mean
Different inhibitor complex
What is the equation for KI’
[ES] [I] / [ESI]
What does the Michaelis Menten equation look like for Uncompetitive inhibition
V0 = Vmax [S] / Km + a’ [S]
What does an uncompetitive inhibitor do to Km
Lowers it
Why is this
The inhibitor is removing some fraction of enzymes from the reaction (only ES removed which shifts binding EQ to right)
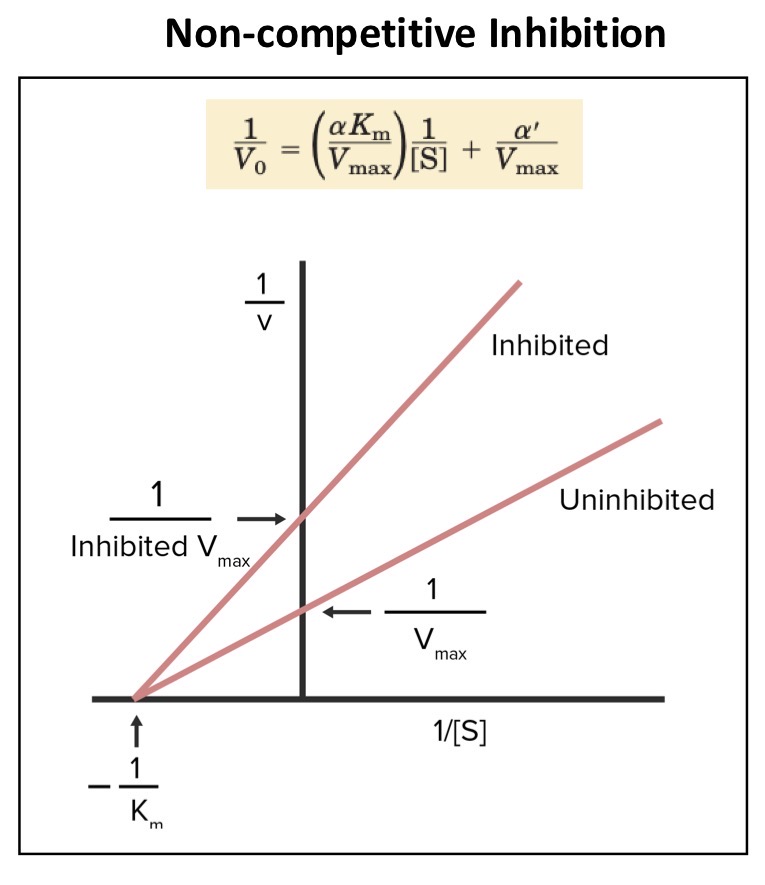
What is the action of a non-competitive inhibitor
Binds to both the free enzyme and the enzyme-substrate complex at a different site to the substrate
The inhibitor alters the structure of the protein in a way that prevents catalysis but doesn’t affect substrate binding
Michaelis-Menten equation for non-competitive inhibitors
V0 = Vmax [S] / aKm + a’[S]
Can the substrate still bind to the EI complex
Yes, but the ESI complex doesn’t progress to product
What does the inhibitor lower the concentration of
The functional enzyme
This therefore decreases
Vmax
What happens to Km
It remains the same as the substrate affinity for the enzyme is unchanged
What is the lineweaver burk plot for competitive inhibitors
Non-competitive?
Uncompetitive?
What does irreversible enzyme inhibitors do
They form permanent covalent interactions with a functional group on the enzyme which is essential for activity
What are mechanism-based irreversible inhibitors
The inhibitor binds like a substrate, catalytic mechanism starts but the catalytic process stalls as the inhibitor-enzyme interactions are too strong
What does it mean for enzyme activity to be spatiotemporally controlled
Means they have to catalyse reactions in the correct space at the correct time
What are the 4 types of regulation
Allosteric regulation
Reversible Covalent Modification
Proteolytic Cleavage
Feedback Regulation
If a product is in excess, how can enzymes be regulated
They can divert resources elsewhere
If a product is in demand, how can enzymes be regulated
They are activated to produce more of the required biomolecule
How do allosteric regulators (modulator/effectors) work
They bond to another site away from the active site on the enzyme, which can either activate or deactivate it through a conformational change
What is the difference between non-competitive inhibitors and allosteric regulators
Non-competitive is always inhibitory, whereas allosteric modulation can both inhibit or activate (doesn’t follow Michaelis-Menten
What is reversible covalent modification (or post-translational modification)
The enzyme activity function is modulated by covalent modification of amino acid residues in an enzyme molecule
What is an example of reversible covalent modification
Kinases add phosphate groups, phosphatases remove them
Enzymes regulates by proteolytic cleave are produced as
Inactive, called zymogen or pro-enzymes
How does it get activated
After removal of a polypeptide segment by proteolytic cleavage which causes conformational changes that form a fully functional enzyme (i.e. revealing active site)
What is feedback regulation
The end-product of an enzymatic pathway inhibits an upstream enzyme to decrease rate of production