Dr. Sata - BIO 311C Final Exam Review (pt. 1)
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
Investigating the molecular structure of DNA in order to understand the chemical basis of inheritance is an example of which approach utilized in the study of biology?
A. Reductionism
B. Systems Biology
C. Emergent Properties
D. Deductive Reasoning
A. Reductionism
Which of the following is the best description of a control for an experiment?
A. The control group is matched with the experimental group except for the one experimental variable
B. The control group is kept in an unchanging eenvironment
C. The control group is left alone by the experimenters
D. The control group is exposed to only one variable rather than several
A. A. The control group is matched with the experimental group except for the one experimental variable
Living things are divided into three different domains. Which of these domains are classified as prokaryotes?
A. Archaea and Eukarya
B. Bacteria and Archaea
C. Bacteria and Fungi
D. Bacteria and Eukarya
B. Bacteria and Archaea
All living things on Earth along with all the places where life exists are collectively described by which of the following terms?
A. The biosphere
B. Population
C. Biological community
D. An ecosystem
A. The biosphere
If you want to design a drug that will easily enter the non-polar biological membrane, which of the following functional groups would be the best to add to such a molecule?
A. Methyl
B. Carbonyl
C. Carboxyl
D. Phosphate
A. Methyl
How much of whole milk (4% fat) is needed to make 100 ml of 1% fat milk by mixing with skim milk to make up the volume?
A. 25 ml
B. 60 ml
C. 20 ml
D. 400 ml
E. 75 ml
A. 25 ml
If an atom of sulfur (atomic number 16 were allowed to react with atoms of hyrdogen (atomic number 1), which of the following molecules would be formed?
H-S-H
Which of the following molecules contains polar covalent bonds?
A. H2
B. H2O
C. CH2
D. O2
B. H2O
The most stable interaction between magnesium (atomic number 12) and chlorine (atomic number 17) forms which of the following molecules?
A. Mg2Cl in which atoms are joined by ionic bonds
B. MgCl in which atoms are joined by ionic bonds
C. MgCl2 in which atoms are joined by covalent bonds
D. MgCl in which atoms are joined by covalent bonds
E. MgCl2 in which atoms are joined by ionic bonds
E. MgCl2 in which atoms are joined by ionic bonds
If acid rain has lowered the pH of a particular lake to pH 4.0, which of the following statements about this lake is true?
The hydroxyl ion concentration is 1 x 10^-10 moles per liter of lake water
If a salamander clings to surfaces through hydrogen bonds, it would have the most difficulty clinging to which of the following surfaces?
A. A surface with a thin film of water
B. A surface with a thin film of vegetable oil
C. A surface coated with a thin film of vinegar (acetic acid)
D. A surface coated with a thin film of ammonia (NH3)
B. A surface with a thin film of vegetable oil
Nitrogen (N) is much more electronegative than hydrogen (H). Which of the following statements about the atoms in ammonia (NH3) is correct?
A. Each hydrogen atom has a partial positive charge; the nitrogen atom has a partial negative charge
B. The nitrogen atom has a full positive charge; each hydrogen atom has a full positive charge
C. Each hydrogen atom has a partial negative charge; the nitrogen atom has a full positive charge
D. There are nonpolar covalent bonds between each hydrogen atom and the nitrogen atom
E. The nitrogen atom has a partial postitve charge; each hydrogen atom has a partial negative charge
A. Each hydrogen atom has a partial positive charge; the nitrogen atom has a partial negative charge
A solution contains 0.0000001 (10^-7) moles of hydroxyl ions (OH^-) per liter. Which of the following best describes this solution?
A. Acidic: H+ donor
B. Basic: H+ donor
C. Neutral
D. Basic: H+ acceptor
E. Acidic: H= acceptor
C. Neutral
One mole (mol) of glucose (molecular mass = 180 daltons) is
A. 180 grams of glucose dissolved in 1 L of solution
B. 180 x 10^23 molecules of glucose
C. the largest amount of glucose that can be dissolved in 1 L of solution
D. 1 kg of glucose dissolved in 1 L of solution
E. 180 grams of glucose
E. 180 grams of glucose
An amino acid such as Glycine will be a Zwitteron (both + and - charges on it) at which of the following conditions?
A. neutral pH
B. acidic pH
C. alkaline pH
D. at all pH ranges
A. neutral
The sequence 5'-GAACGA'3' may be found in which of the following?
A. DNA only
B. RNA only
C. either DNA or RNA
D. neither DNA nor RNA
C. either DNA or RNA
How many peptide bonds are present in a polypeptide that contains 45 amino acids?
A. 44
B. 46
C. 45
D. 90
A. 44
Bonds formed between the side chains (R groups) in a polypeptide are most important in stabilizing which of the following?
A. secondary structure
B. quaternary structure
C. tertiary structure
D. primary structure
C. tertiary structure
A carbon skeleton is covalently bonded to both an amino group and carboxyl group. When placed in water, it
A. will function as both an acid and a base
B. will function only as a base because of the amino group
C. will function only as an acid because of the carboxyl group
D. will function as neither an acid nor a base
A. will function as both an acid and a base
Hydroxyl groups are the primary chemical group associated with a particular biologically important macromolecule. Which of the following statements regarding this macromolecule is true?
A. it will likely dissolve in nonpolar solvents
B. it will likely form hydrogen bonds with water
C. it is likely to have a planar structure
D. it is unlikely to dissolve in water
B. it will likely form hydrogen bonds with water
The molecular formula for glucose is C6H12O6. What would be the molecular formula for a molecule made by linking three glucose together by dehydration reactions?
A. C18H30O15
B. C6H10O5
C. C18H36O18
D. C18H32O16
E. C16H36O16
D. C18H32O16
A nucleotide is composed of
A. a nitrogenous base, a phosphate group, and a pentose sugar
B. a nitrogenous base, a phosphate group, a pentose sugar, and an amino acid C. a nitrogenous base and a pentose sugar
D. a nitrogenous base and a phosphate group
A. a nitrogenous base, a phosphate group, and a pentose sugar
Lactase is an enzyme composed of a single polypeptide that hydrolyzes the disaccharide lactose to produce monosaccharides. The optimal pH for lactase activity is 6. Transfer of lactase to pH 5 results in a substantial decrease in enzyme activity, likely due to the disruption of:
A. the secondary and tertiary structures of the enzyme
B. only the primary structure of the enzyme
C. the secondary, tertiary, and quaternary structures of the enzyme
D. the primary and secondary structures of the enzyme
A. the secondary and tertiary structures of the enzyme
Testosterone and estradiol are male and female sex hormones, respectively, in many vertebrates. How do these molecules differ from each other?
A. Testosterone and estradiol are cis-trans isomers but have the same molecular formula
B. Testosterone and estradiol are structural isomers but have the same molecular formula
C. Testosterone and estradiol have different functional groups attached to the same carbon skeleton
D. Testosterone and estradiol are enantiomers of the same organic molecule
C. Testosterone and estradiol have different functional groups attached to the same carbon skeleton
Which of the following statements about the 5' ends of RNA polymers is correct?
A. the 5' ends have nitrogenous bases attached to the number 5 carbons of ribose
B. the 5' ends have hydroxyl groups attached to the number 5 carbons of ribose
C. the 5' ends have phosphate groups attached to the number 5 carbons of ribose
D. the 5' ends have phosphate groups attached to the number 5 carbons of the nitrogenous base
C. the 5' ends have phosphate groups attached to the number 5 carbons of ribose
A protein was degraded to three random fragments and the individual fragments were sequenced. The following is a collection of three such fragments with overlapping amino acid sequences. Align them in such a way as to find out the correct protein sequence. Note the protein sequences always start with MET (methionine). (hint: look for overlapping sequences) |
1. GLU-MET-PHE-TYR-SER-GLN-VAL|
2. MET-GLY-VAL-LEU-SER-GLU-MET-PHE-TYR
3. TYR-SER-GLN-VAL-GLY-TRP-ASN-PRO-VAL
MET-GLY-VAL-LEU-SER-GLU-MET-PHE-TYR-SER-GLN-VAL GLY-TRP-ASN-PRO-VAL
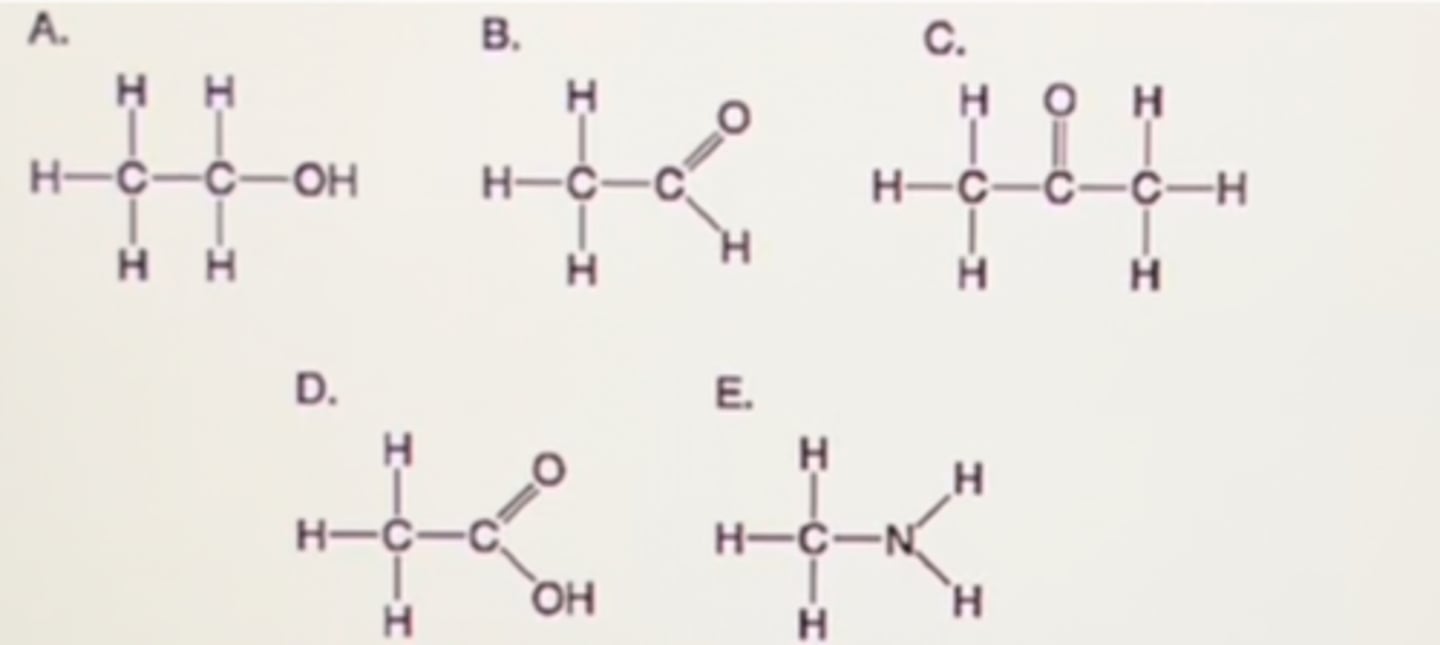
Which molecule shown in Figure 3.5 would have a positive charge in a cell?
E. H _ H
H-C-N_
H H
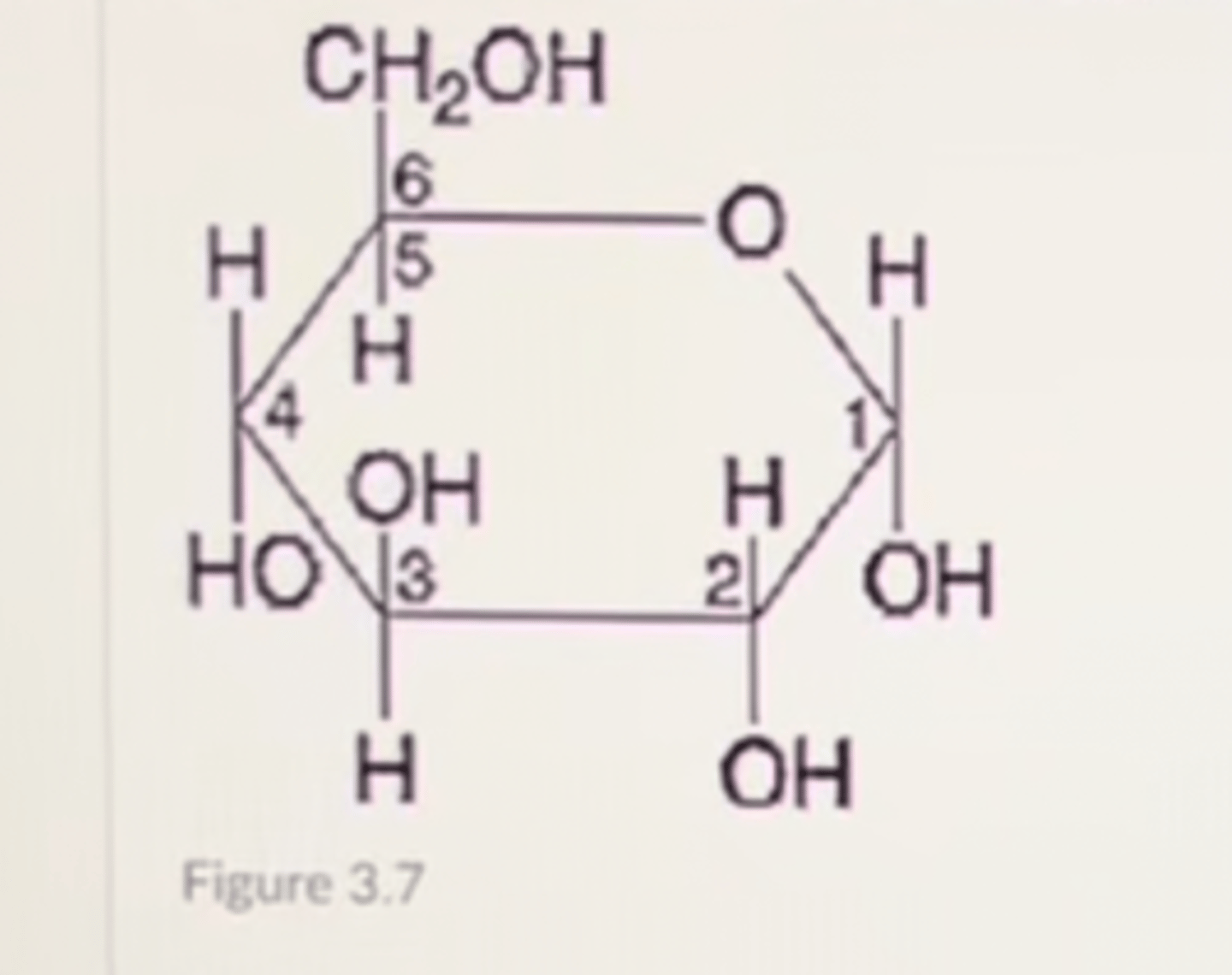
If two of the molecules shown in Figure 3.7 are linked together, carbon-1 of one molecule to carbon-4 of the other, the bond that is formed would be found in which of the following polymers?
A. glycogen
B. chitin
C. nucleic acid
D. polypeptide
E. cellulose
A. glycogen
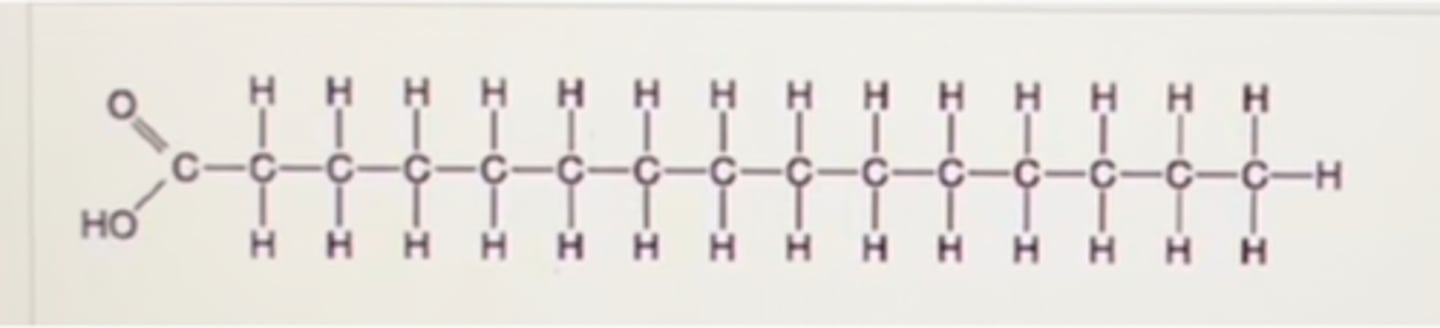
Which of the following statements regarding the molecule illustrated in Figure 3.8 is true?
A. Molecules of this type are usually liquid at room temperature.
B. It would be highly soluble in water.
C. It Is an entirely nonpolar molecule
D. It is a saturated fatty acid.
D. It is a saturated fatty acid.
Which molecule(s) shown in Figure 3.5 is (are) ionized in a cell?
A. A only
B. D only
C. D and E
D. E only
E. B and D
C. D and E
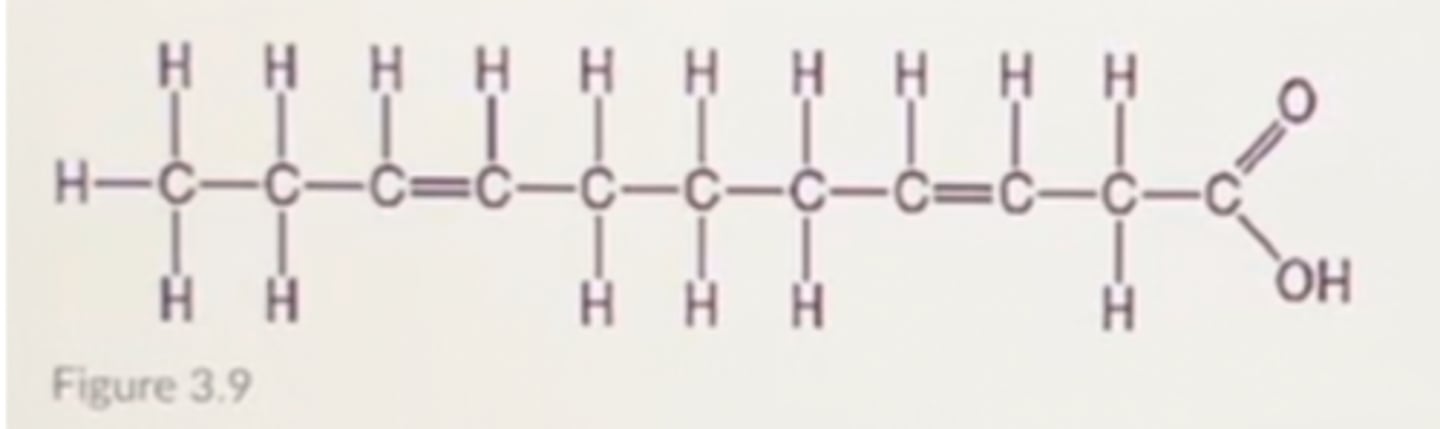
Which of the following statements regarding the molecule illustrated in Figure 3.9 is true?
A. It is a saturated fatty acid
B. it is highly soluble in water.
C. It is an entirely nonpolar molecule.
D. Molecules of this type are usually liquid at room temperature.
D. Molecules of this type are usually liquid at room temperature.