ANHB3323 (18) - stem cells
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
Embryonic stem cell
All multicellular organisms arise from one cell
most commonly from inner cell mass of blastocyst
Types:
Pluripotent (inner cell mass)
differentiate ONLY into embryonic cells
Totipotent (blastomere)
can differentiate into placental and embryonic cells
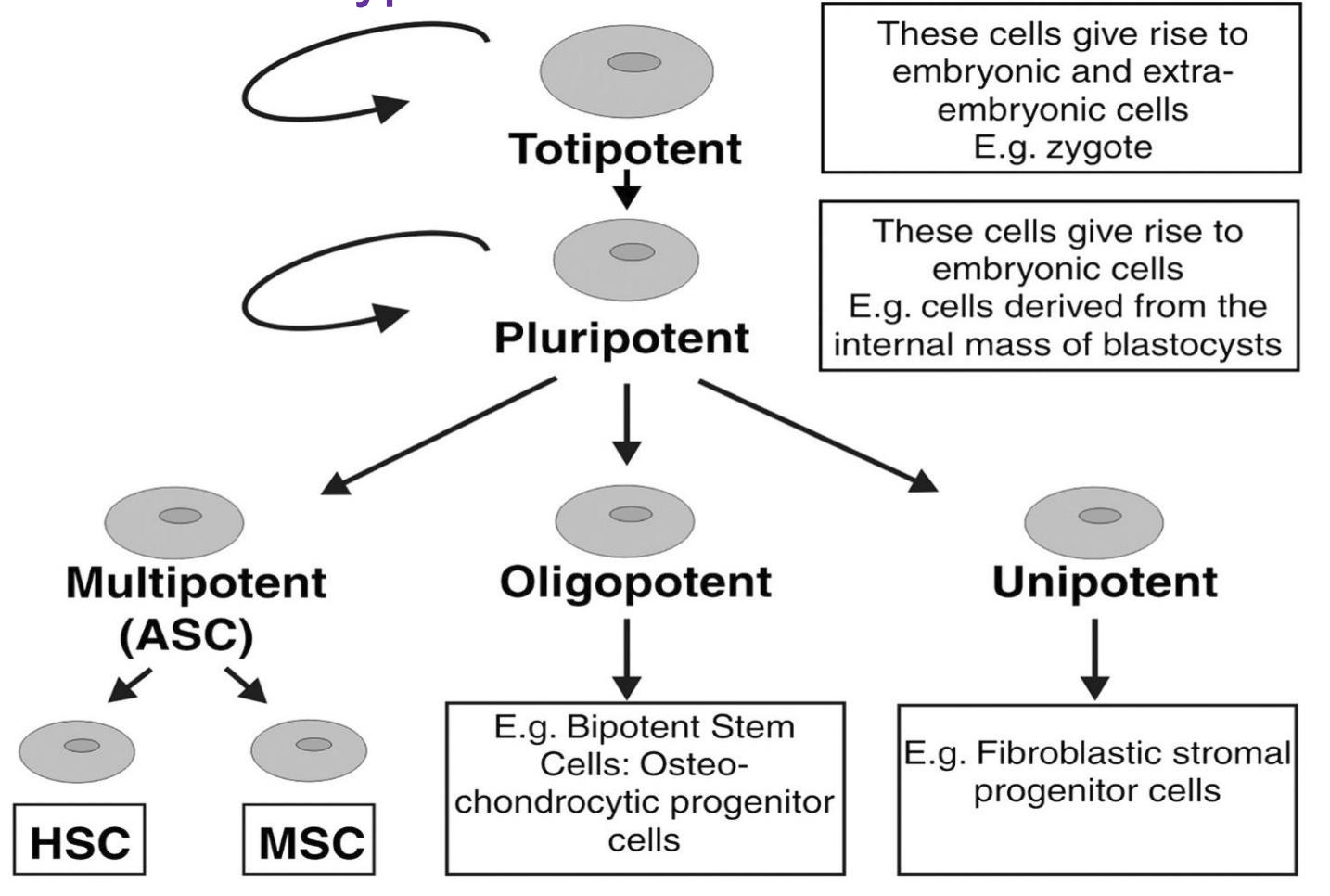
Adult stem cell
replace damaged or dead cells
Types:
Multipotent
Oligopotent
Unipotent
[limited differentiation potential)
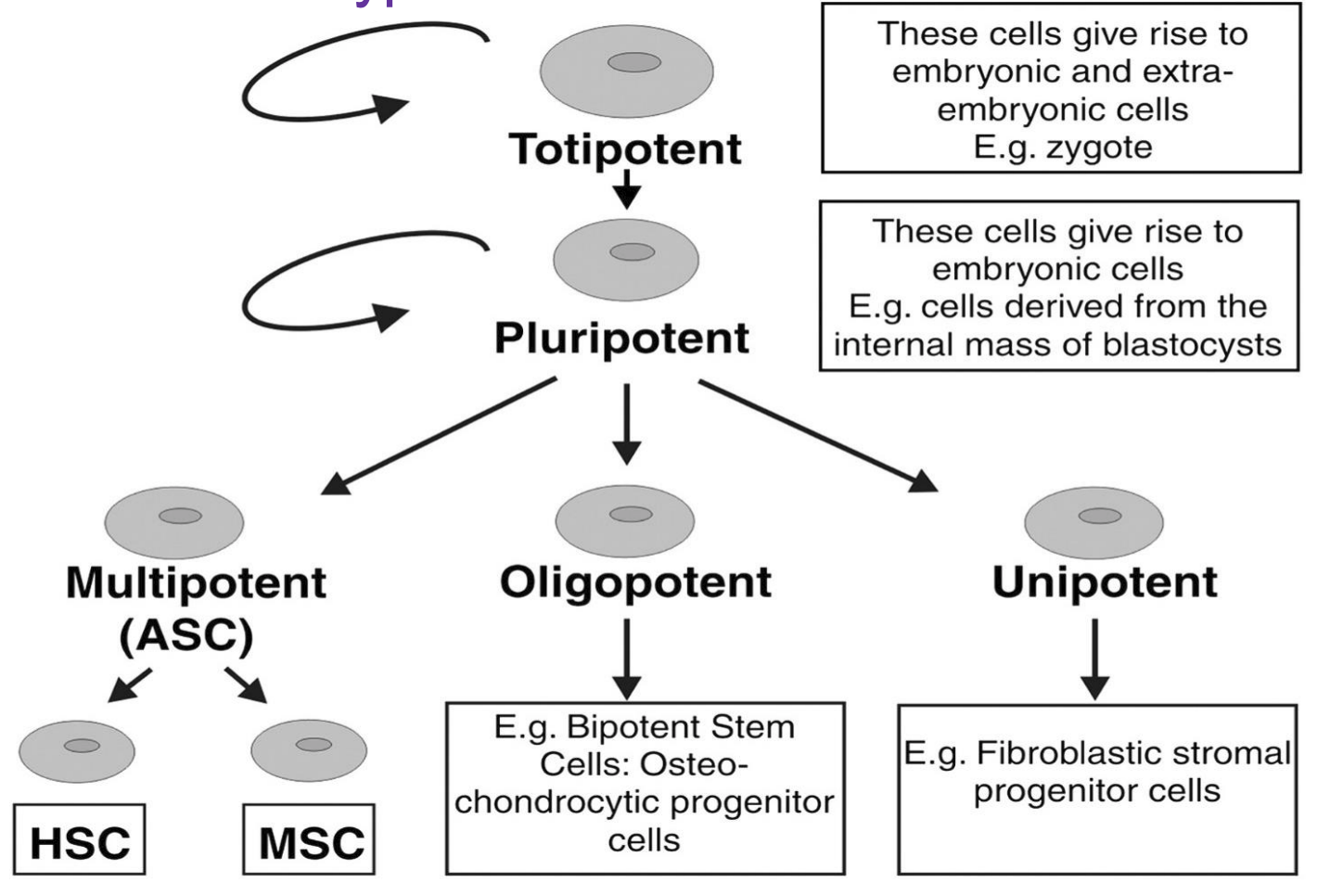
Types of self renewal (stem cells)
symmetric self-renewal = creates cells that are the same as original
asymmetric self-renewal = creates original cell + another cell that is slightly more restricted
symmetric differentiation = creates 2 cells that are more restricted
Extraction of embryonic stem cells
from inner cell mass of blastocyst = kills embryo (controversial)
Induced pluripotent stem cells
reprogramming fully differentiated adult cells
= embryonic stem cell like properties
Timeline of stem cell discovery
1. 1962 = frog cloned
2. 1983 = produced multinucleate cells
3. 1987 = trancription factors + mammalian cell cloning
4. 1996 = mammal cloned
5. 1998 = mice cloned
6. 2001 = human embryo cloned
7. 2006 = induced pluripotent stem cells generated
8. 2010 = AID pluripotentcy regulator
Enbryonic stem cell uses (5)
1. study in tissue cultures = to understand factors controlling cell differentiation
2. source of human cells for transplantation = tissue engineering
3. cloning
4. somatic cell nuclear transfer
5. induced pluripotent stem cells (IPSCs)
Somatic Cell nuclear transfer (5 general steps)
1. Egg + somatic cell
2. Egg anucleated
3. Somatic cell nucleus isolated
4. Egg + somatic cell combined
5. Gives rise to totipotent cell
Generates autologous cells (patient’s own cells) which can avoid the immune rejection problem
Why is cloning in humans unacceptable? (4 reasons)
1. ethics
2. Genes vs environment
3. status/quality of DNA ('aged')
4. role of maternal cytoplasmic factors and mtDNA
Bivalent Domains
chromatin regions that contain both activating and repressive marks
= maintains embryonic stem cells in 'poised' state
Adult stem cell uses (3)
1. tissue engineering ex vivo (spare parts)
2. Tissue repair by cell therapy
3. Genetically correct autologous cells = treat disease
Examples of tissue repair by adult stem cell therapy (4)
1. Haematopoetic cells = leukaemia
2. Islet cells = diabetes
3. Heart = after heart attack
4. Neurodegenerative disorders = parkinsons etc.
stem cell niche
environment in which they reside
= dominant part in regulating stem cell activity and behaviour
(adult stem cells are all in a niche)
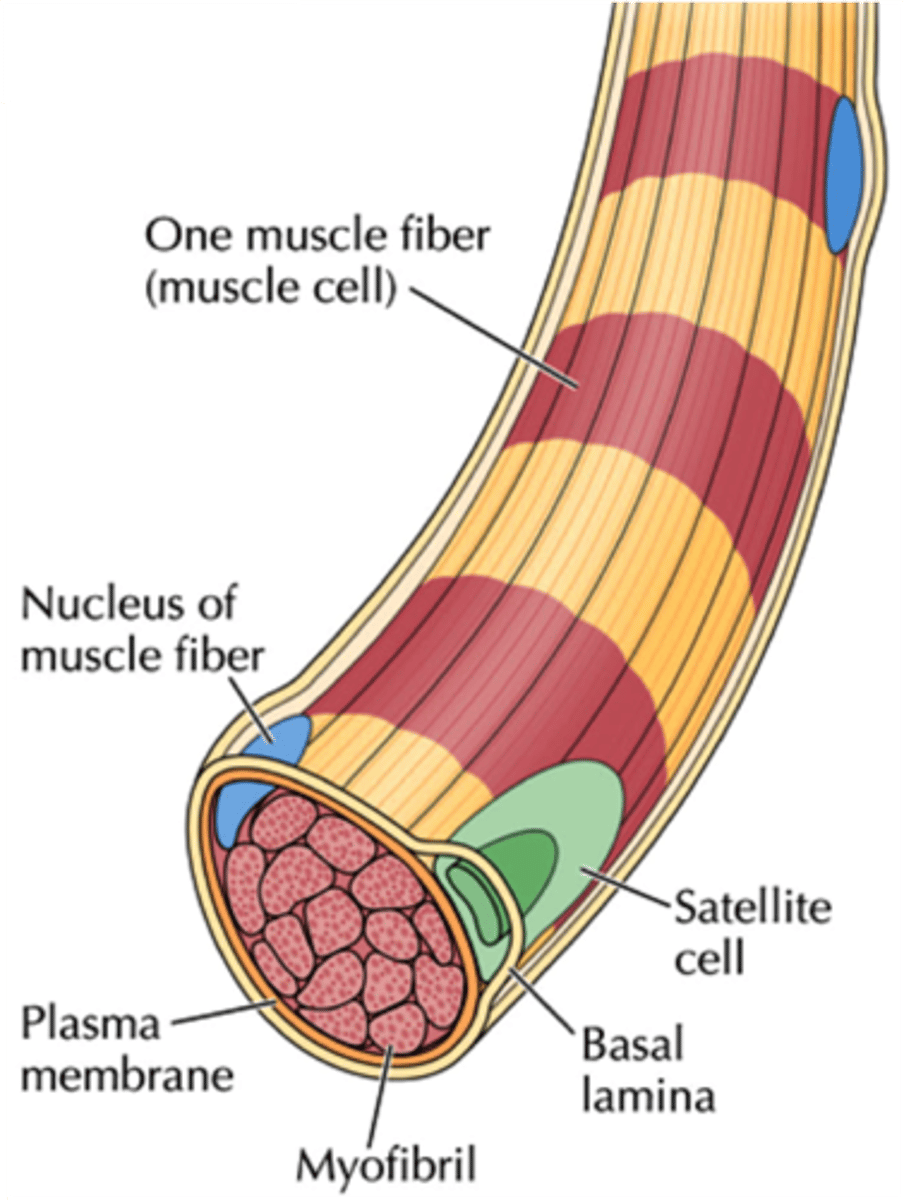
Satellite cells (location and function)
next to plasma membrane under basal lamina (in skeletal muscle)
activated by external stimuli = differentiate together + upregulation of MyoD
upregulation of MyoD = differentiates myoblast → myotube
Haematopoietic stem cells
give rise to many blood cells
(found in bone marrow)
2 regions of the body that undergo Neurogenesis
1. Olfactory Bulb (smell)
in Subventricular zone (SVZ) of lateral ventrical
2. Hippocampus (learning/memory)
in Subgranular zone (SGZ) of dentate gyrus, in hippocampus
Induced pluripotent stem cell usecase
Direct reprogramming in vivo via viruses/other molecules
Uses:
disease modelling
pharmacological screening + toxicity testing (drug development)
transplanting cells back into patient
Benefits of iPSCs
1. reduced ethical concenrns
2. disease understanding/treatment
3. patient specificity