OCR A-level chemistry - module 2
1/105
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
106 Terms
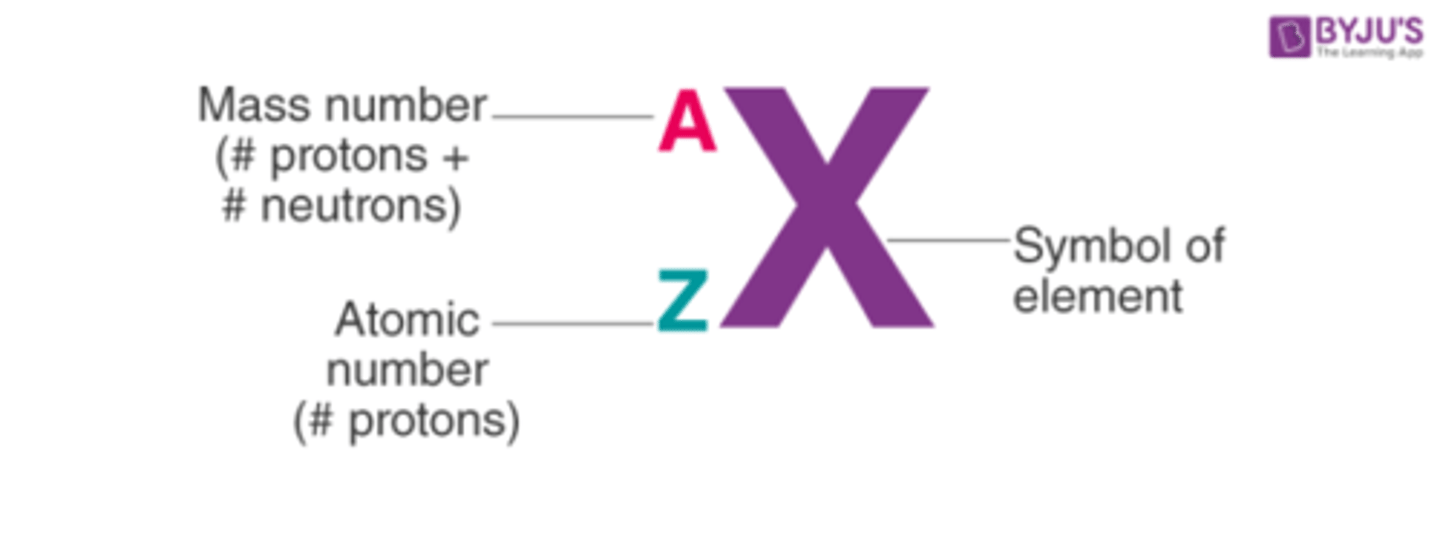
nuclear symbols
top/bigger no = RELATIVE ATOMIC MASS (-AN=neutrons)
bottom/smaller no = ATOMIC NUMBER (total proton/electrons)
ions
- diff number of protons and electrons
- negative ion = more electrons
- positive ion = less electrons
e.g F = 9 protons
so F^- = 10 electrons
overall charge = 9 - 10 = -1
Mg = 12 protons
so Mg^2+ = 10 electrons
overall charge 12 - 10 = +2
define 'isotopes'
isotopes of an element are atoms with the same number of protons but different numbers of neutrons
(same atomic number, different mass number)
why do Isotopes have similar chemical properties?
they have the same configuration of electrons
why do isotopes have slightly different physical properties? e.g densities and rates of diffusion
physical properties depend more on the mass of the atom
define 'relative atomic mass'
Ar is the weighted mean mass of an atom of an element compared to 1/12th of the mass of an atom of carbon-12
define 'relative isotopic mass'
the mass of an atom of an isotope of an element compared to 1/12th of the mass of an atom of carbon-12
how do you calculate relative atomic mass Ar?
sum of (isotope abundance x isotope mass number) / 100 (sum of abundances of all the isotopes)
3 applications of a mass spectrometer
1. drug analysis
2. forensic analysis
3. carbon dating
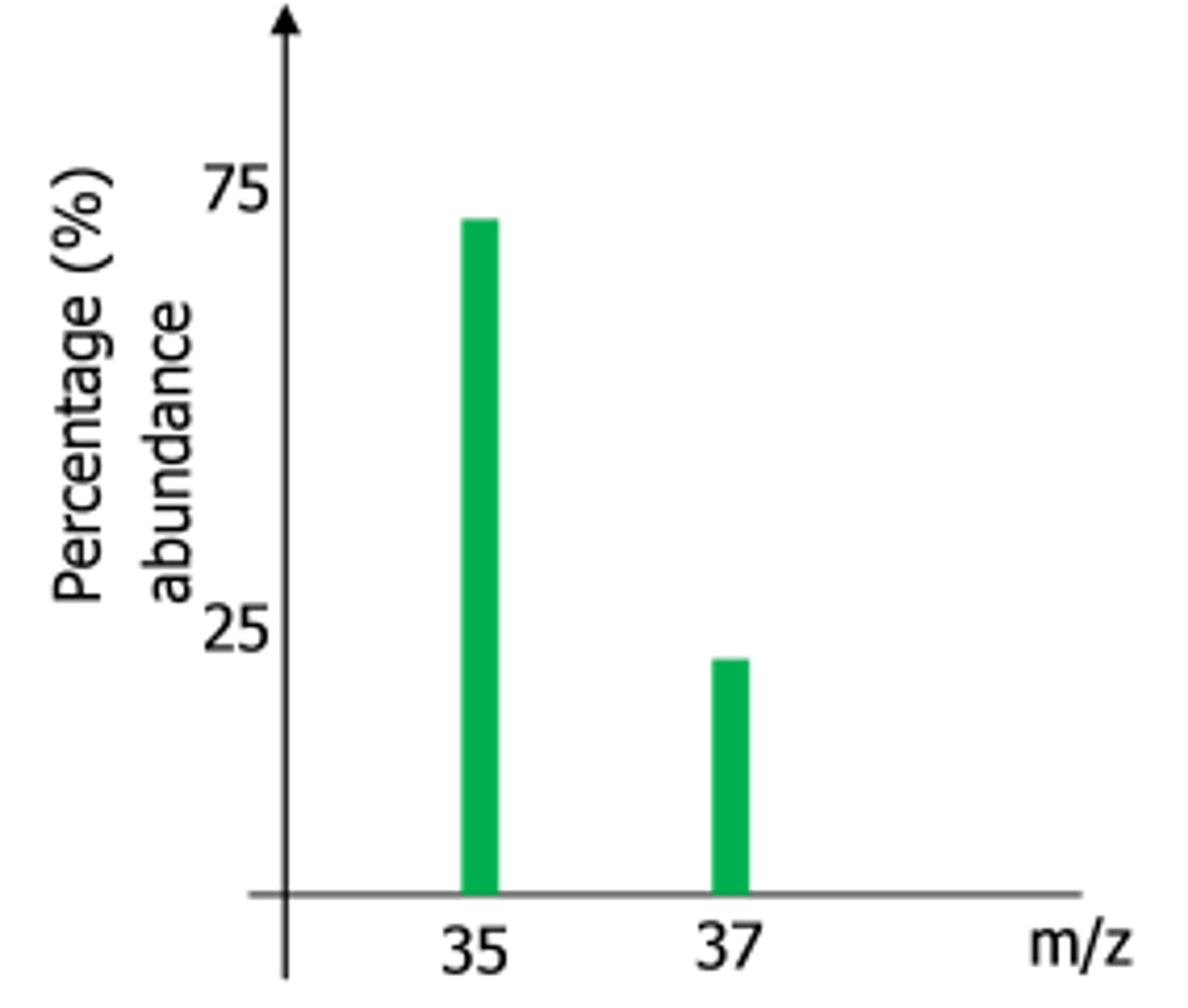
how to read a mass spectrum
y-axis = abundance of ions (%), peak of each element = relative isotopic abundance
x-axis = relative isotopic mass
- sum of multiplying each mass by % abundance
- divide by 100 (should be sum of abundances)
= RELATIVE ATOMIC MASS
e.g (35 x 75) + (37 x 25) / 100
= 35.5 (RAM of chlorine)
what if the relative abundances are not as a %
- sum of multiplying each mass by its abundance
- divide by the SUM of relative abundances
how to calculate % abundance in a table
(relative abundance of isotope / sum of all relative abundance of isotopes) x 100
one mole of a substance is
6.02 × 10^23 particles (Avogadro's constant)
formula for finding the moles from the number of atoms or molecules
number of moles = number of particles/6.02 x 10^23
what is molar mass?
the mass of one mole of a substance, has the same numerical value as the Mr, just add g mol-1
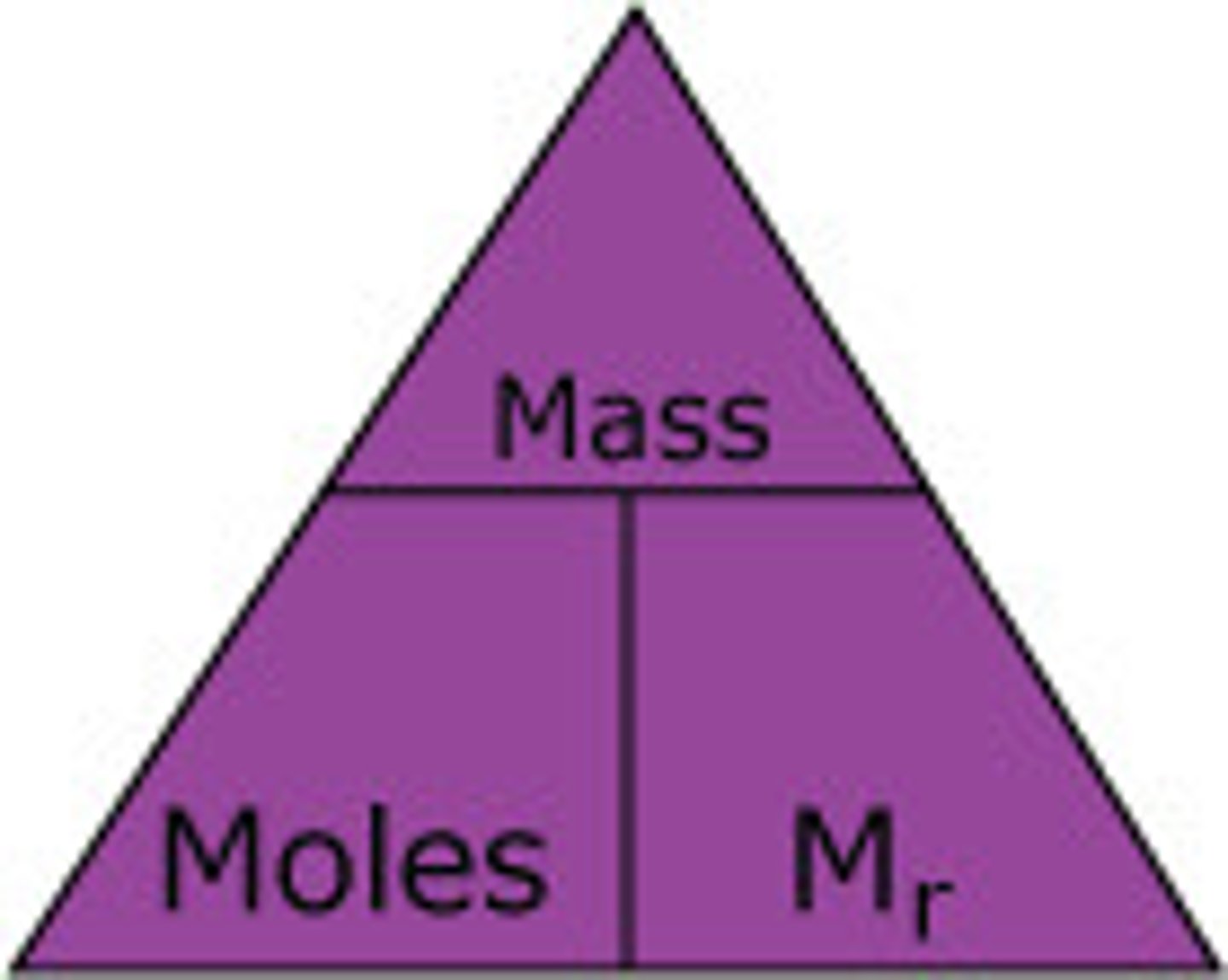
formula incl moles, mass and Mr
moles = mass/Mr
how do you find the number of molecules, particles or atoms in the sample from the moles?
moles x (6.02x10^23) = number of molecules
then x by how many atoms for atoms
e.g H2S = 3 atoms
m3 to dm3
dm3 to cm3
m3 to cm3
1. x1000
2. x1000
3. x1000,000
what is room temperature and pressure?
298K and 100kPa
formula for number of moles at room temp and pressure
moles = volume in dm3 / 24
or vol in cm3 / 24 000
what is the ideal gas equation?
pV = nRT
pV = nRT units
p = pressure (Pa)
V = volume (m3)
n = moles
R = the gas constant (8.314 JK-1 mol-1)
T = temperature (K)
degrees celcius to kelvin
+273
kPa to Pa
x1000
cm3 to m3
divide by 1,000,000
dm3 to m3
divide by 1000
what is the relationship between pressure and volume?
pressure is inversely proportional to volume P ∝ 1/V
as P decreases V increases, and as P increases V decreases
what is concentration and its units?
how many moles/grams of something are dissolved per 1dm3 of solution
mol dm-3 (when w moles)
or g dm-3 (when w mass)
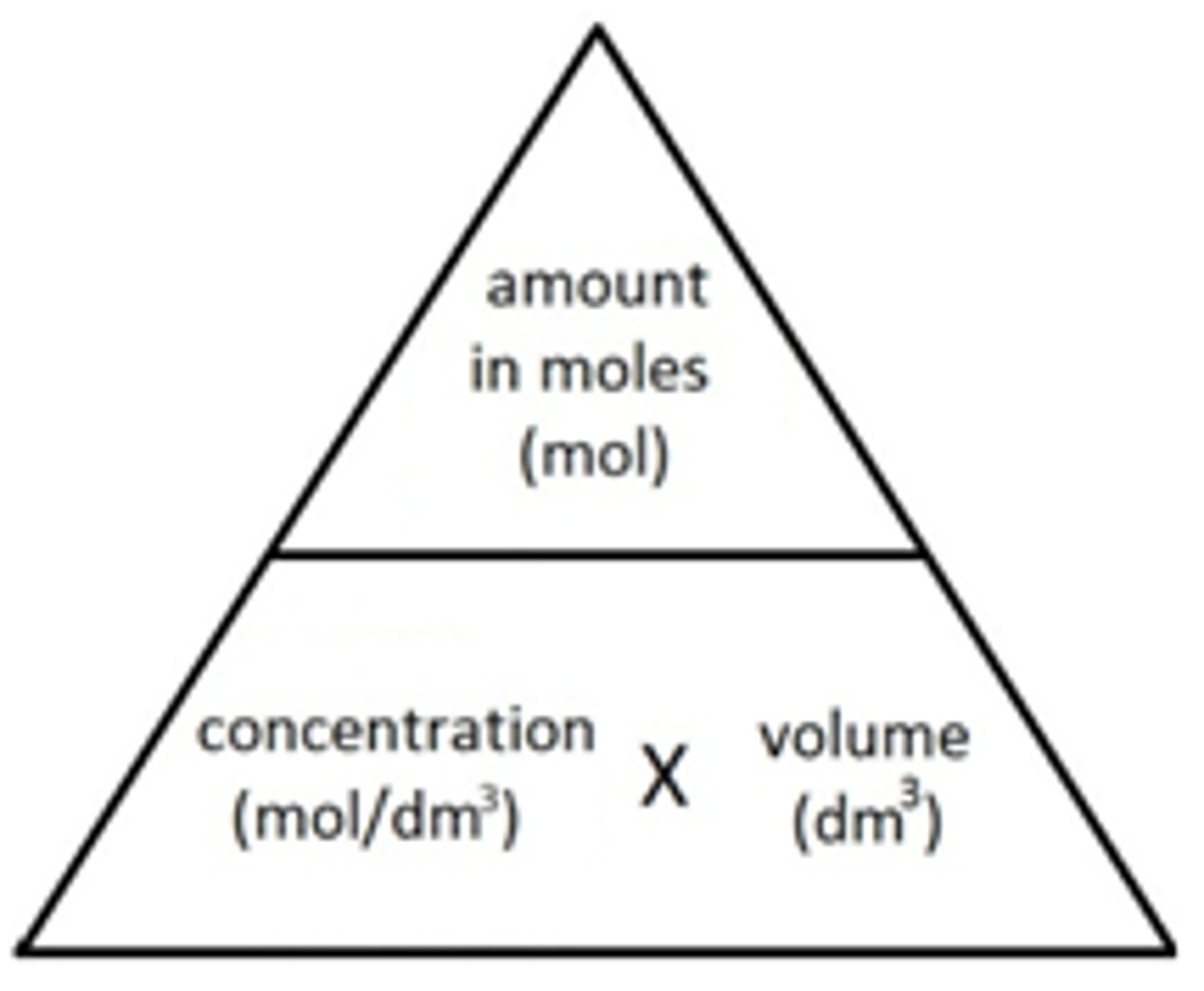
formula icl moles, concentration and vol in dm3
moles = concentration mol dm-3 x vol dm3
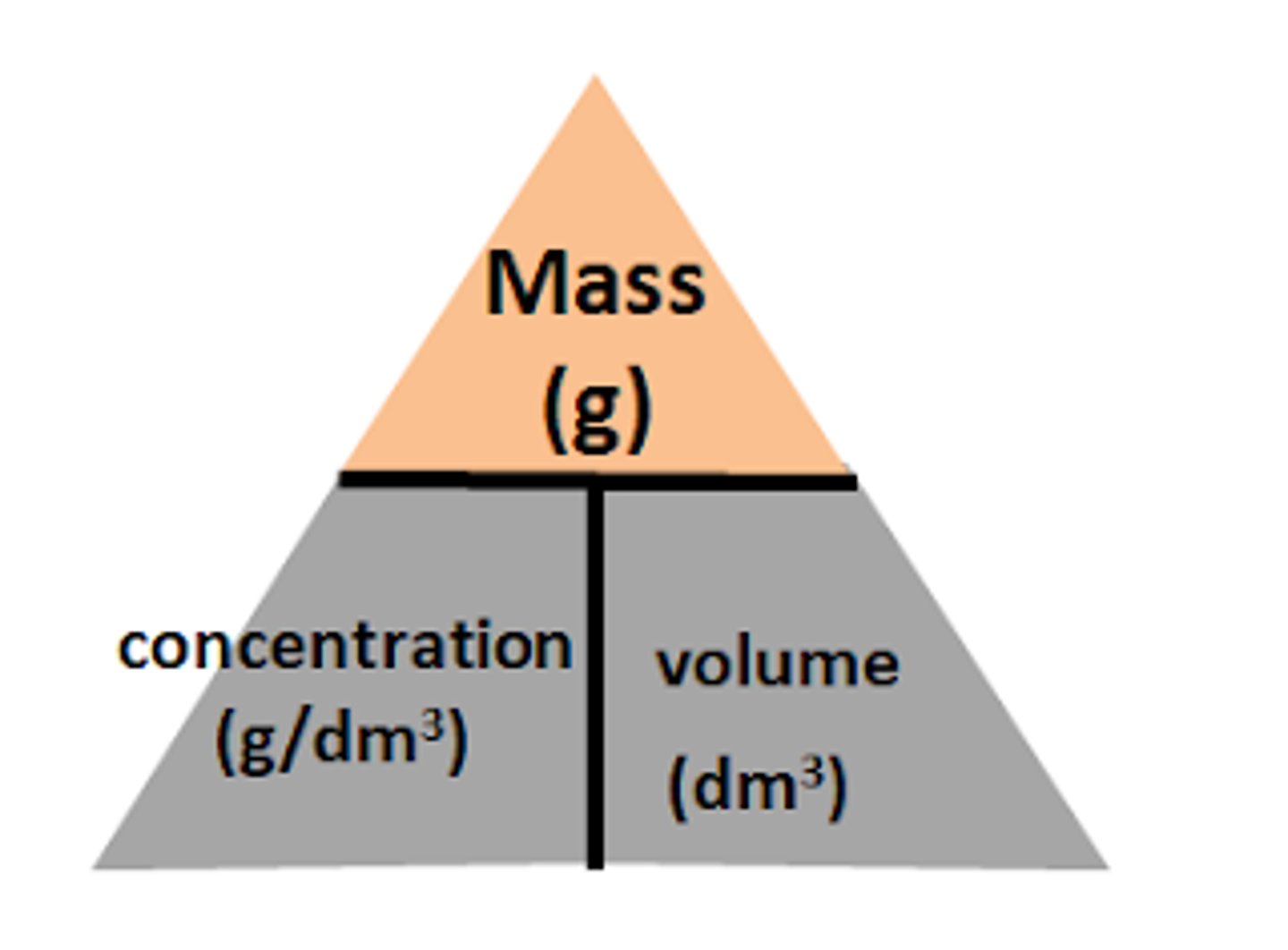
formula icl mass, concentration and vol in dm3
mass = concentration g dm-3 x vol dm3
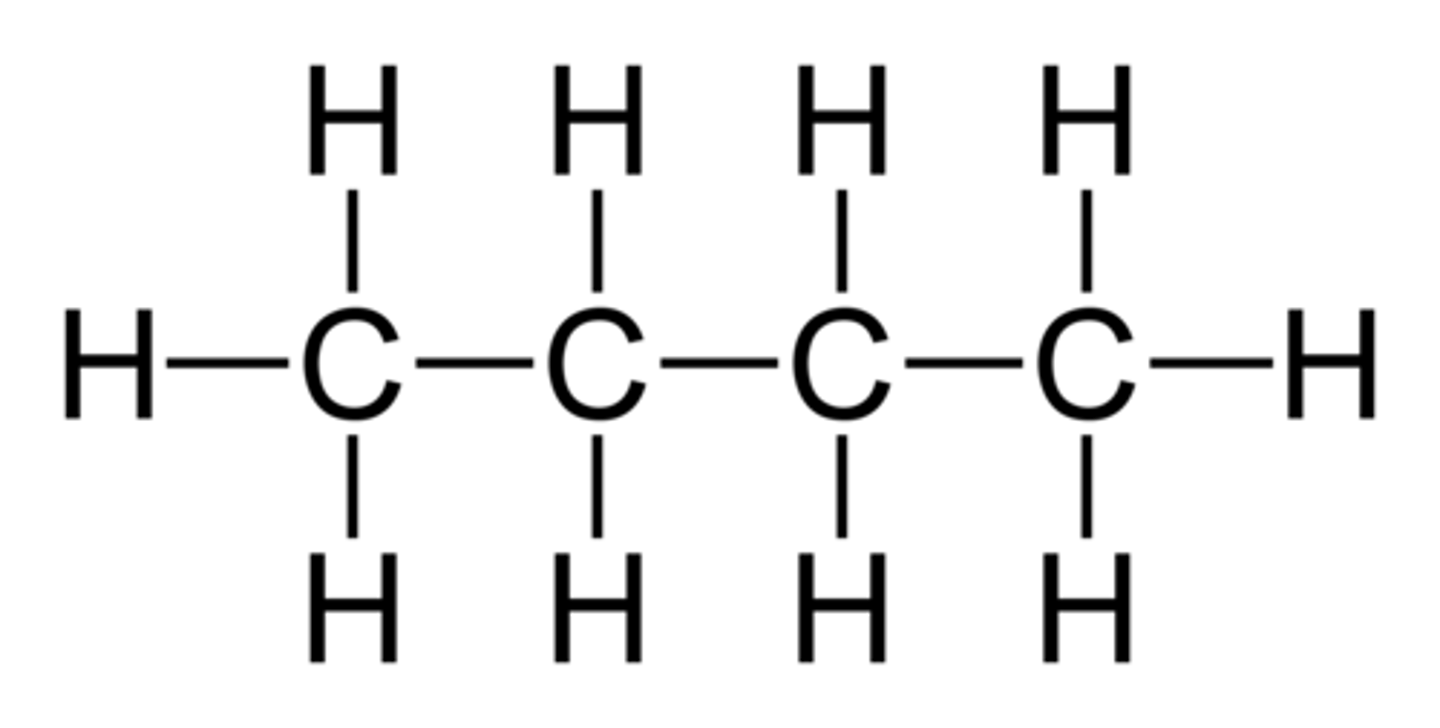
difference between empirical and molecular formula e.g butane
molecular formulas tell you how many atoms of each element actually are in a compound, and empirical formulas tell you the simplest or most reduced ratio of elements in a compound.
e.g butane
mf = C4H10
ef = C2H5, so ratio of carbon to hydrogen atoms is 2:5
how do you calculate the molecular formula of a compound when you know the empirical formula and molecular mass?
1. find empirical mass (Mr)
2. divide molecular mass (sometimes given if diff) by empirical mass = how many empirical units in the molecule
3. molecular formula = empirical formula X that number
how do you calculate empirical formulas from experimental data (mass) e.g hydrocarbon from CO2 and H2O
1. use n=g/Mr to find how many moles of each product has been made
2. use moles to figure out how many moles of each atom u started with
e.g for hydrocarbon
CO2 = 0.10 moles so 0.10 moles of C
H20 = 0.10 moles so
2 moles of H = 0.10 x 2 = 0.20 moles
3. write this as a ratio
C:H = 0.10:0.20
4. divide both sides by the smallest number (0.10) = 1:2
5. so empirical formula of this hydrocarbon is CH2
the mass of the products =
the mass of the reactants
how do you calculate empirical formulas from percentage compositions
1. assume u have 100 g of the compound, change all %s into g
2. use n=g/Mr to find the moles of each element
3. divide each of the moles by the smallest number of moles
4. this tells you the ratio and empirical formula
how do you calculate the molecular formula when you ONLY know the molar mass and mass
1. find the empirical formula using the steps from before (using mass)
2. find the MR of the empirical formula e.g C2H6O
(12x2)+(6)+16 = 46 g
3. divide the molar mass of the alcohol (what ur finding) by this
e.g 92 g mol-1 / 46 = 2
4. multiply empirical formula by this to get the molecular formula
e.g C2H6O x 2 = C4H12O2
formula of ionic compounds e.g potassium sulfate
balance charges
e.g K group one = K+ = +1
SO42- = -2
so for every sulfate ion, you need 2 K ions to = 0
K2SO4
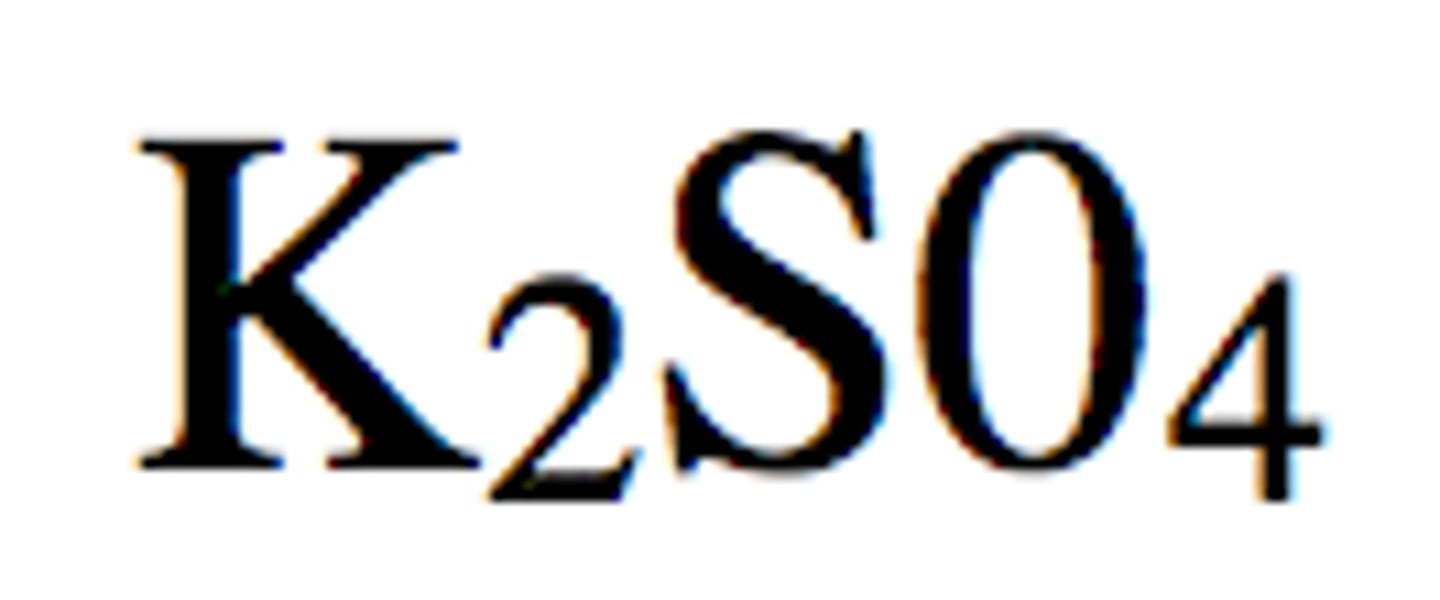
how do you work out ionic equations
1. make sure the equation is BALANCED
2. break up aqueous compounds (ionic) into their ions with charges (swap and drop)
3. cross out spectator ions
4. check charges on each side are balanced
how do you calculate masses of products (or reactants)
1. write out balanced equation
2. work out how many moles you have from the given mass
3. molar ratio of given : what you're working out
4. work out the moles of what you're working out
5. find the mass using n x rfm
how do you calculate gas volumes
1. work out moles using n = m/mr
2. use molar ratio
3. number of moles x 24 = vol in dm3
how do u determine the charge on ions
their group on the periodic table e.g Na group 1 so Na+
S group 6 so S2-
nitrate ion
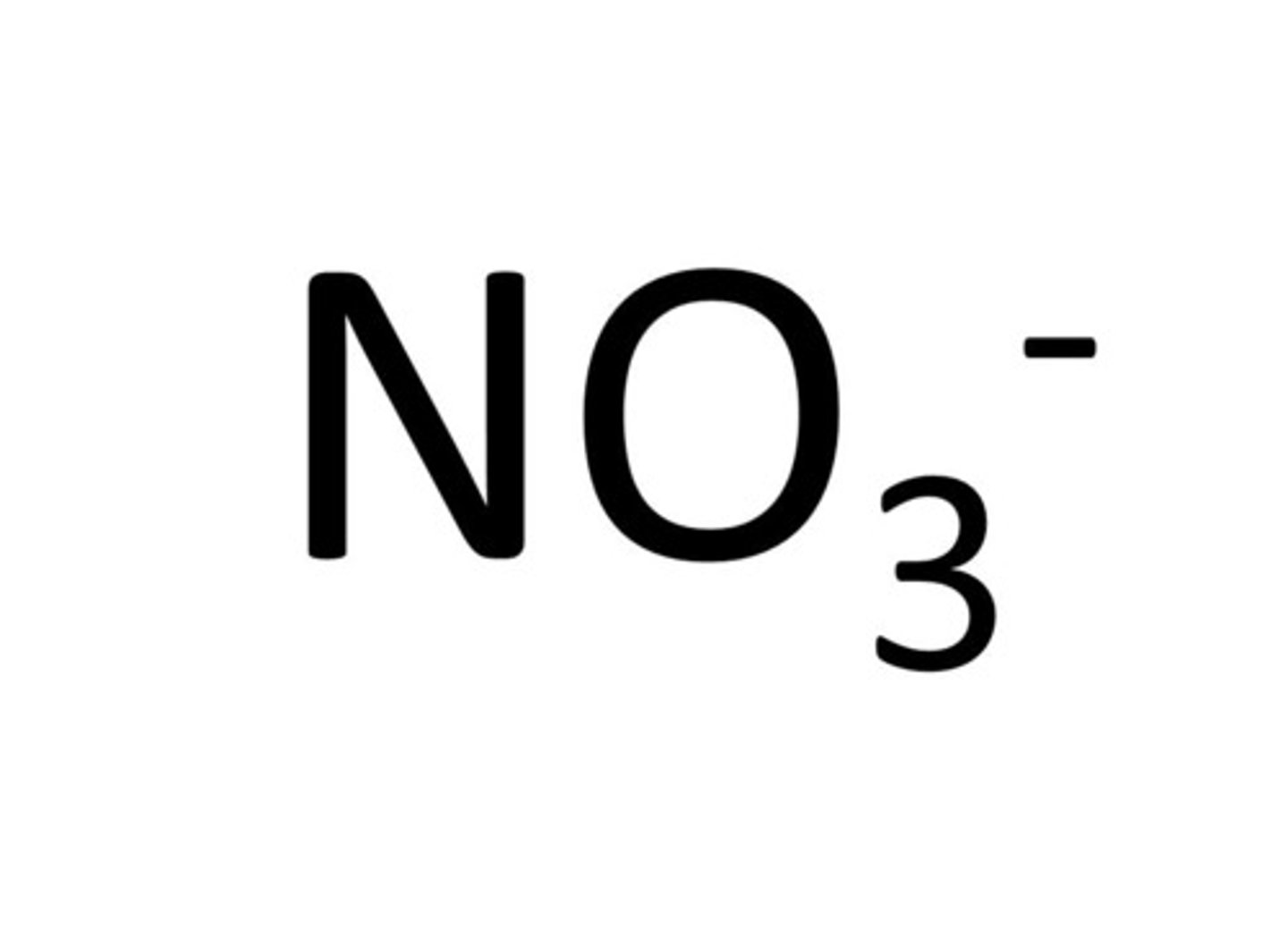
carbonate ion
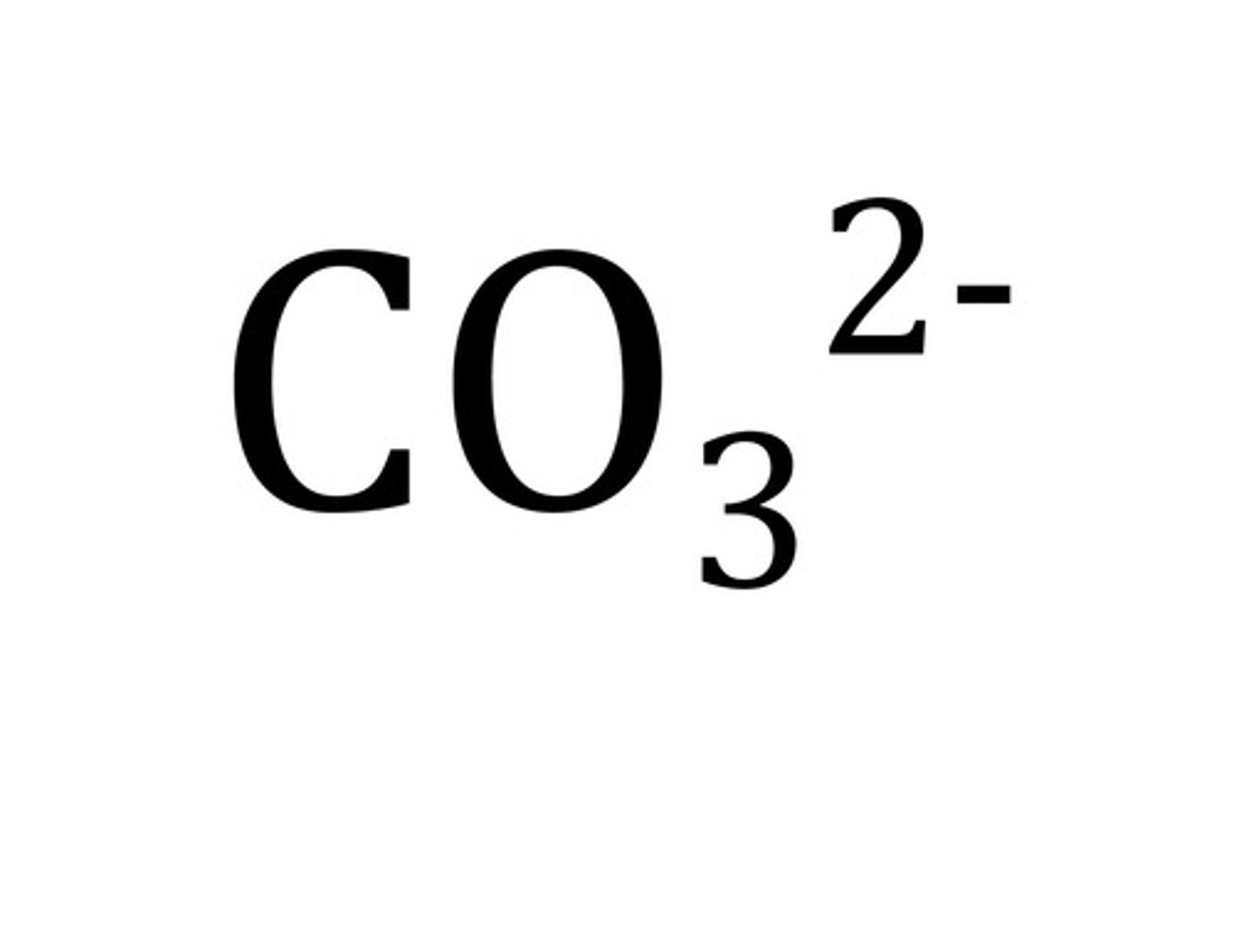
sulfate ion
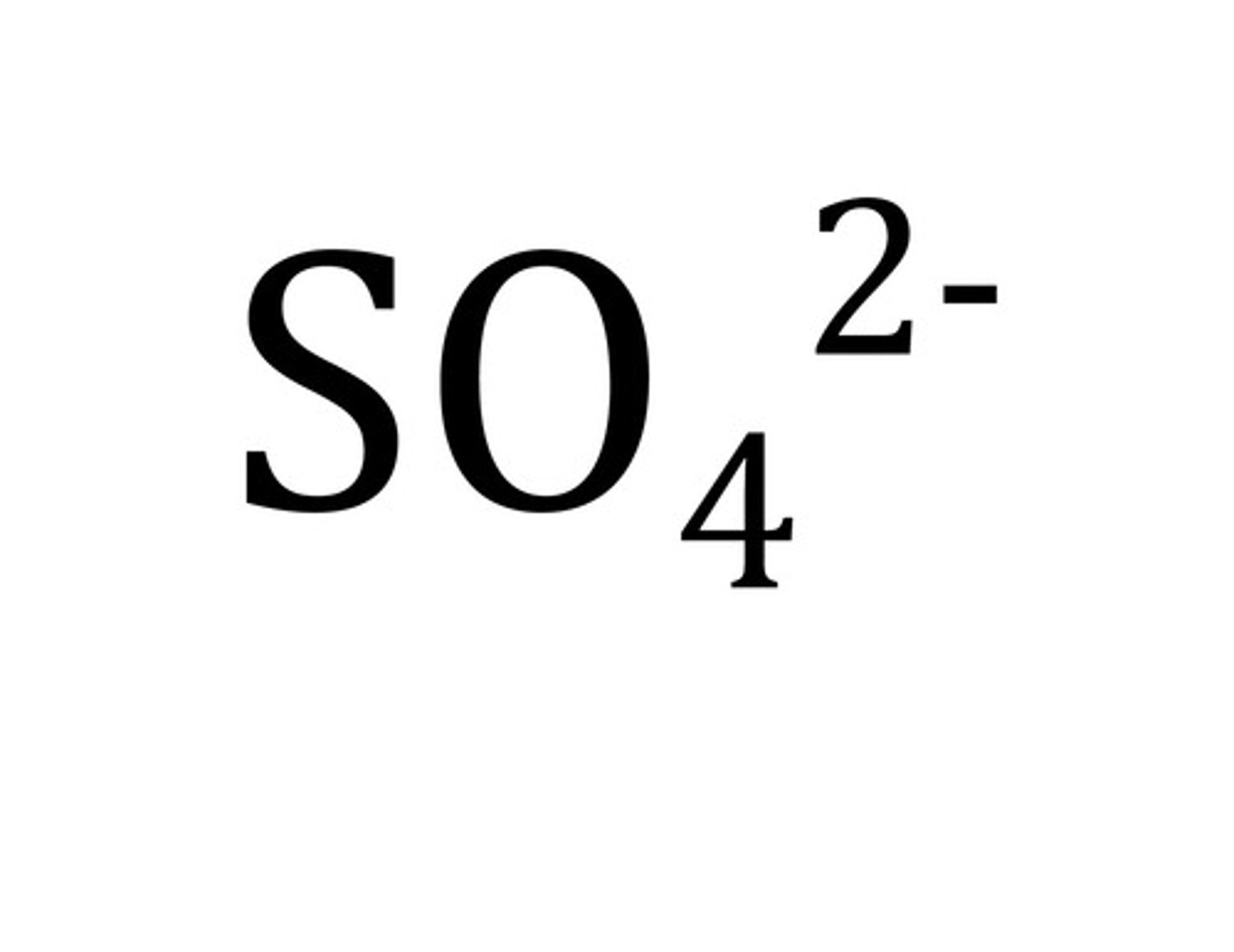
hydroxide ion
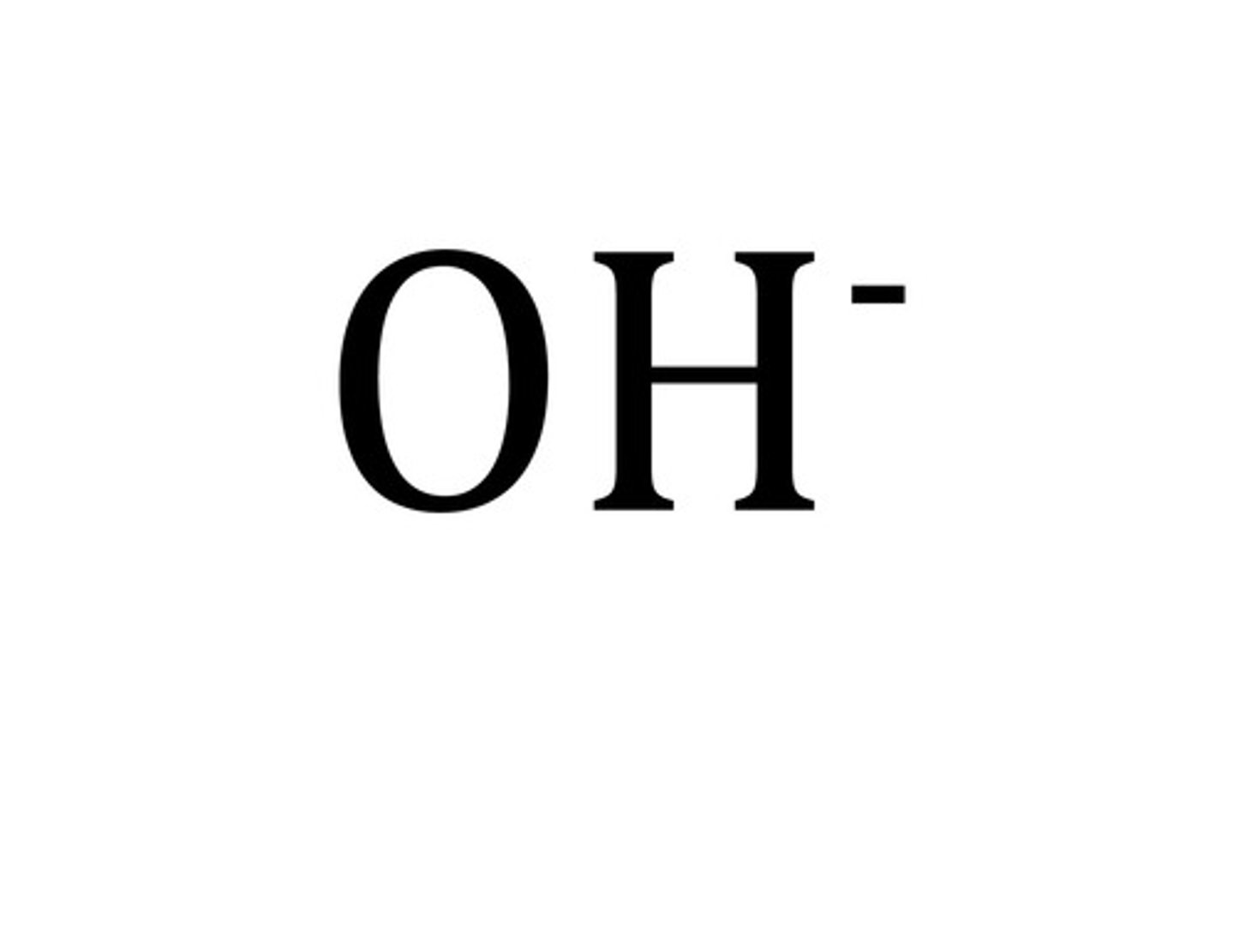
ammonium ion
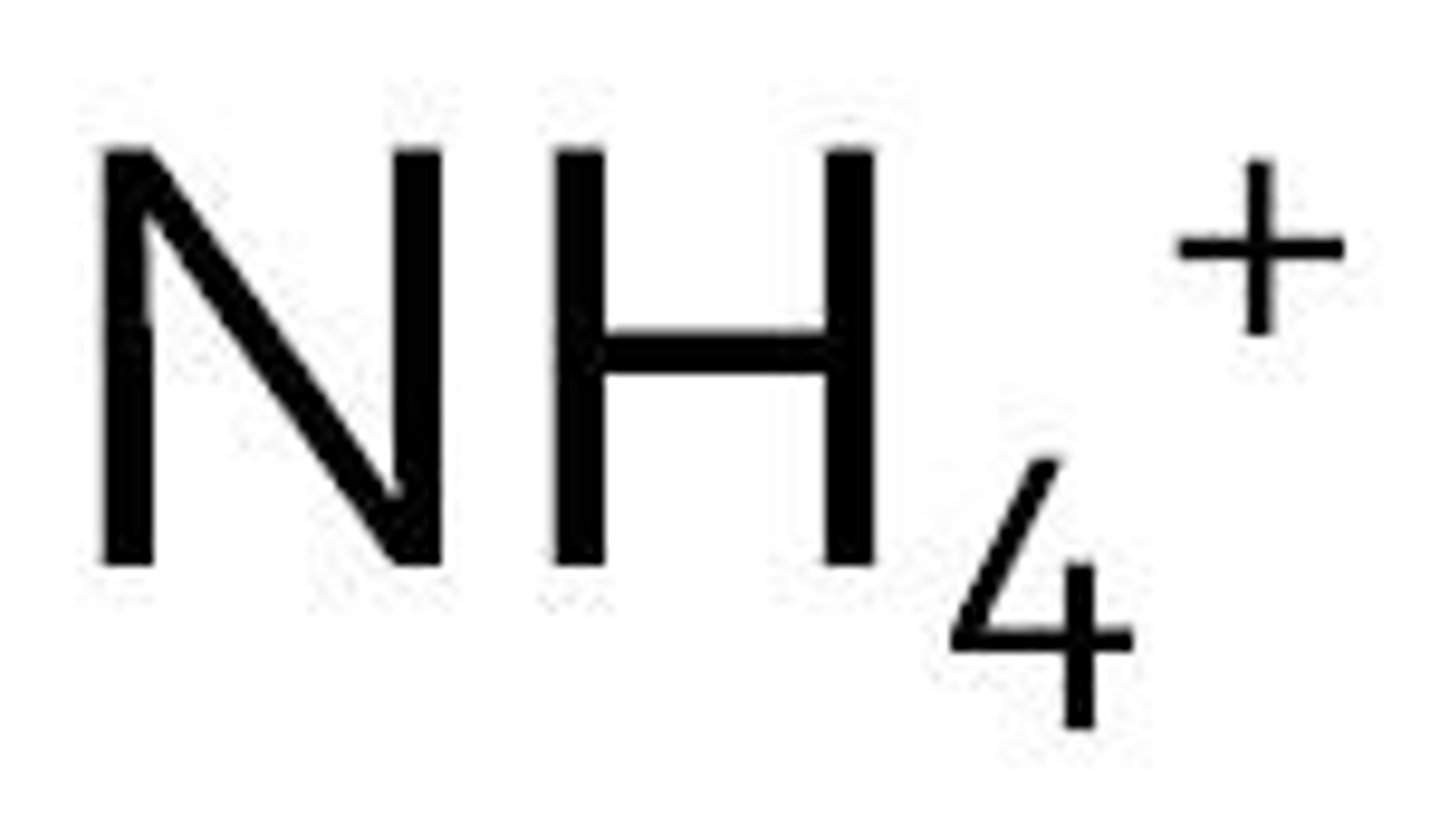
zinc ion
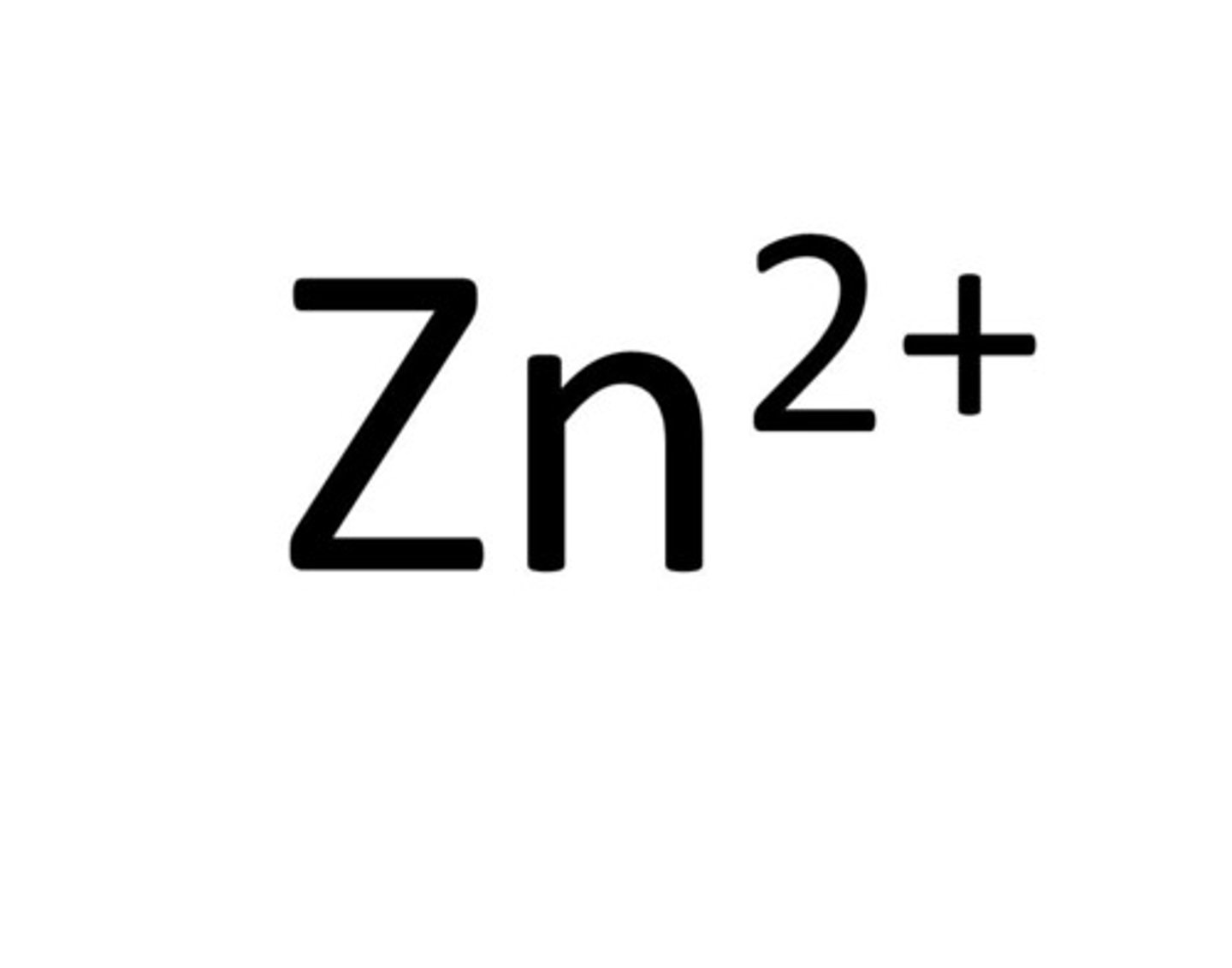
silver ion
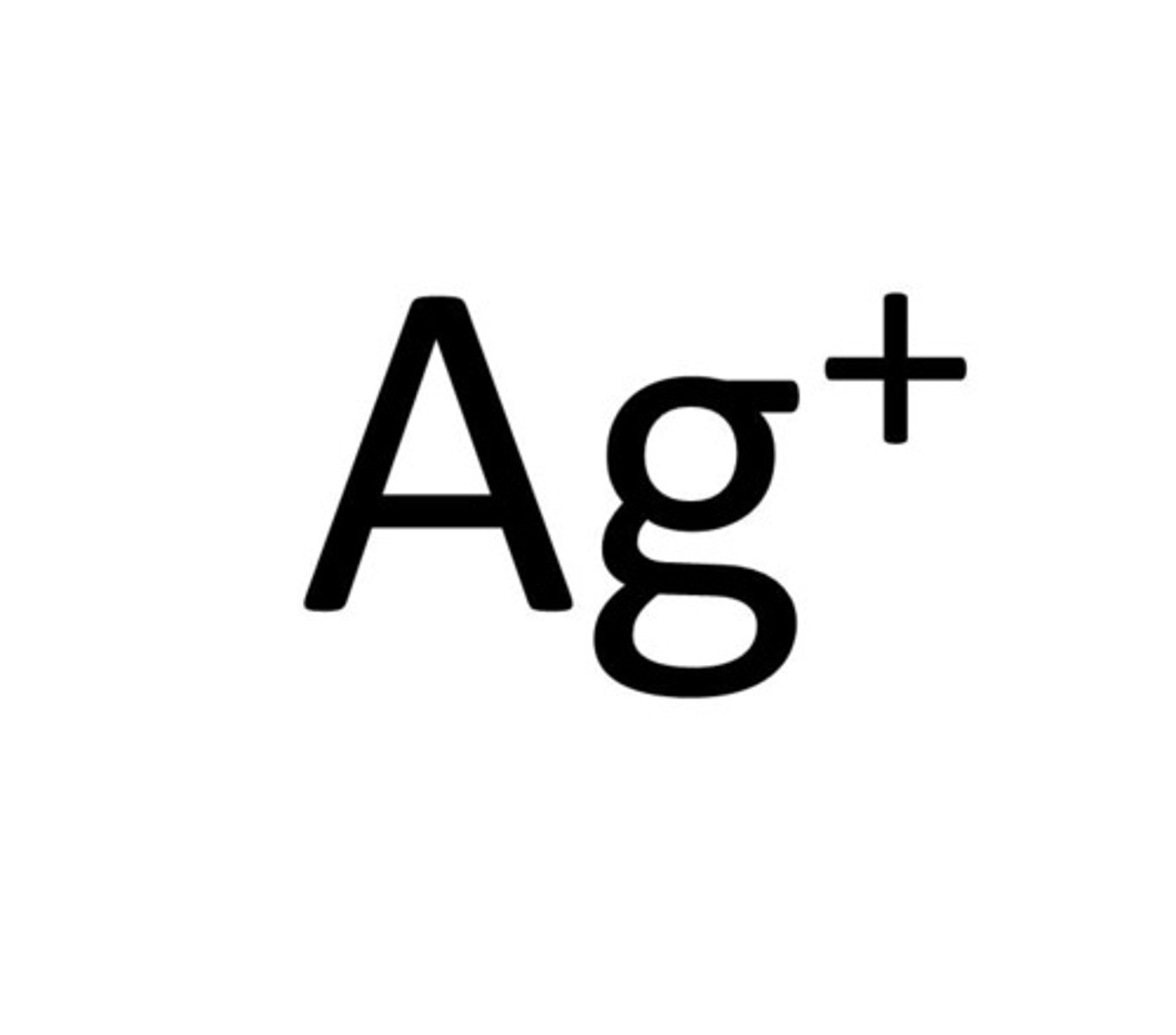
phosphate ion
what is theoretical yield
the mass of the product that should be formed in a chemical reaction assuming no chemicals are 'lost' in the process
how do you calculate theoretical yield
1. work out how many moles of limiting reactant you have using n=g/r
2. ratio this to work out how many moles of the product u expect (usually same)
3. calculate mass of product using g=nxr
how do you calculate % yield
actual yield/theoretical yield X 100
what is atom economy
a measure of the proportion of reactant atoms that become part of the desired product in the balanced chemical equation
how do you calculate % atom economy
RFM of desired product / RFM of all products X 100 (make sure equation is balanced)
define acids
proton donors
release H+ ions when dissolved in water
define bases
proton acceptors
releases OH- ions when dissolved in water
define alkalis
soluble bases
examples of strong acids
hydrochloric acid HCl
sulfuric acis H2SO4
nitric acid HNO3-
phosphoric acid H3PO4
examples of weak acids
ethanoic acid C2H5OH
carbonic acid H2CO3
citric acid
examples of bases
most oxides, all hydroxides and all carbonates AND ammonia
sodium hydroxide NaOH
ammonia NH3
potassium hydroxide KOH
example of an alkali
sodium carbonate
the salts
-chloride
-sulfate
-nitrate
-citrate
-phospate
metal + acid
salt + hydrogen
metal oxide + acid
salt + water
metal hydroxide + acid
salt + water
metal carbonate + acid
salt + carbon dioxide + water
ammonia + acid
salt + water
what is water of crystallisation
the water contained in a crystal lattice
what is a hydrated salt?
a solid salt containing water of crystallisation
what is an anhydrous salt?
a salt which does not contain water of crystallisation
one mole of a particular hydrated salt always has the same number of ...
moles of water of crystallisation, it's formula shows how many
e.g: CuSO4 . 5 H2O
hydrated copper sulfur has 5 moles of water for every mole of the salt
(the dot shows they care not joined by a covalent bond)
how to find the formula of hydrated salts from the mass of the salt when hydrated and anhydrous
1. first find the mass of water lost by taking the mass of the anhydrous salt away from the mass of the hydrated
2. find the number of moles of water lost using that mass and n=g/r
3. find the number of moles of anhydrous salt that is produced using given mass then n=g/r
4. work out the ratio of moles of anhydrous salt to moles of water
5. scale up or down so the ratio is in the form 1 : n then round up and boom!
what if water is given as %
assume 100g of hydrated salt and convert all %s into masses (g)
why do we perform titrations
to find out exactly how much acid is needed to neutralise a quantity of alkali
how do we perform a titration
- first, do a rough titration to get a rough idea of where the end point is
- to do this take an initial reading to see how much alkali is in the burette to begin with
- add the acid to the alkali, giving the flask a regular swirl
- stop when the indicator shows a permanent colour change, END POINT
- record final reading from the burette
- now do an accurate titration
- run the acid in to within 2cm3 of the end point, then add an acid drop wise using the burette
- stop when the indicator shows a permanent colour change, END POINT
- work out the volume of acid used to neutralise the alkali by subtracting the initial reading from the final reading
- this volume is the TITRE
- repeat the titration 3 times
- readings should be concordant (within 0.1cm3 of eachother)
- wash the conical flask between each titration
- then calculate a mean, ignoring anomalous results
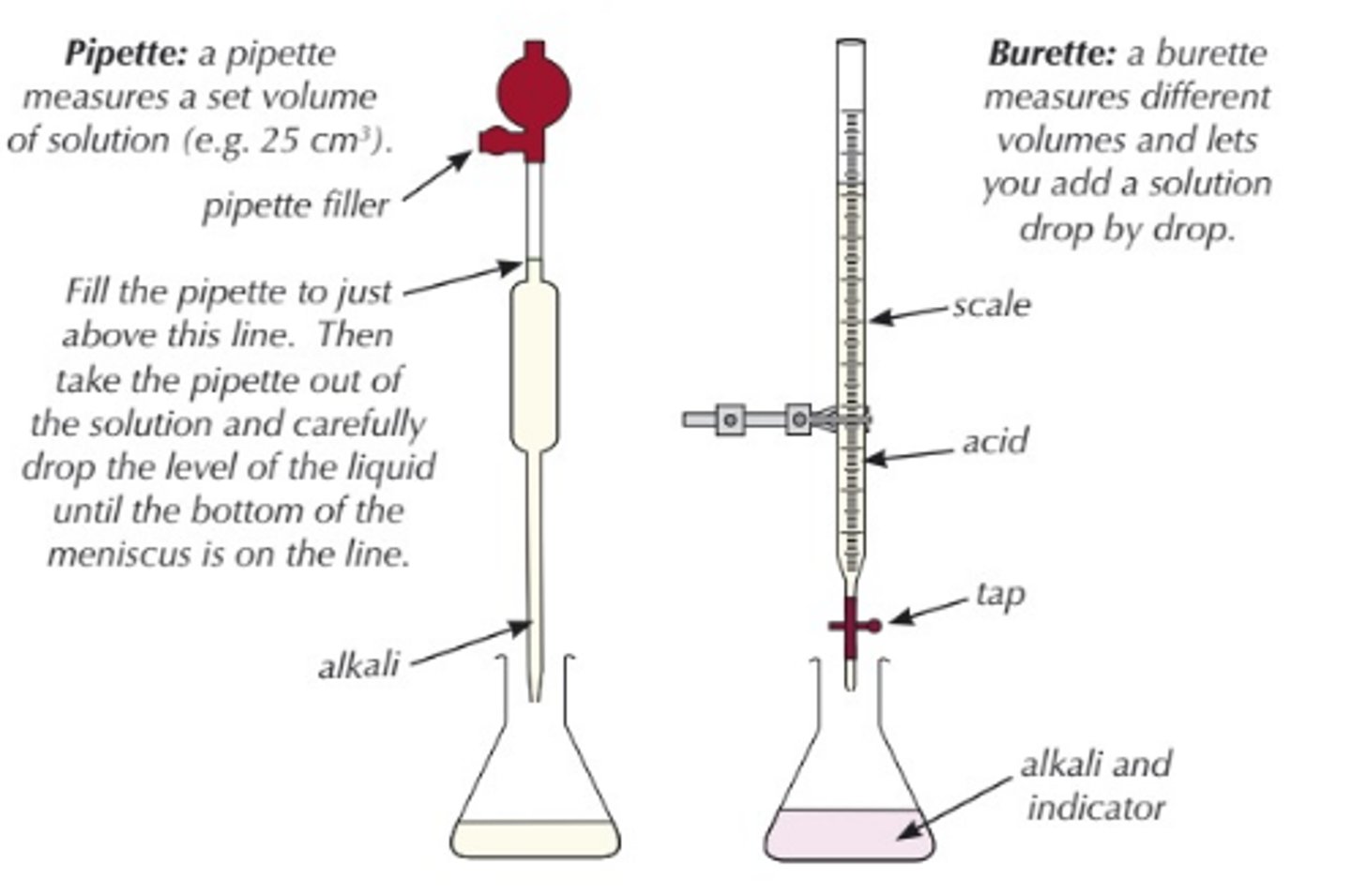
why do we wash the conical flask between each titration
to remove any acid or alkali left in it
methyl orange
turns from YELLOW to RED when adding acid to an alkali

phenolphthalein
turns from PINK to COLOURLESS when adding acid to alkali

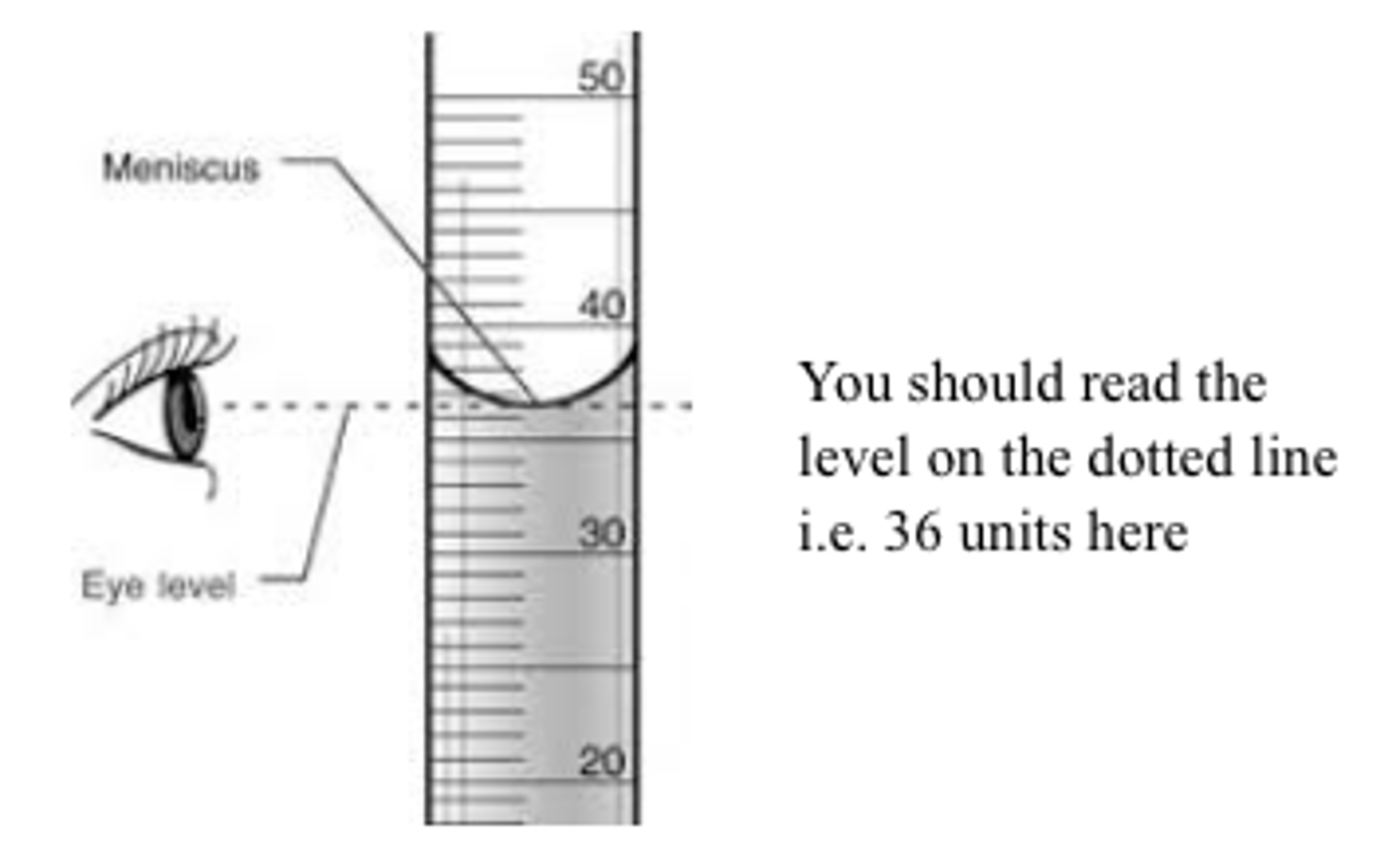
when taking reading from a burette you should take readings from ....
the bottom of the meniscus
why are measuring volumes for satndard solutions or initial vols, using pipette and volumetric flasks more accurate rather than a burette
they only measure a fixed volume e.g 10cm3
when filling these
be at EYE-LEVEL with the GRADUATION LINE and fill exactly up to it
what is a standard solution
a solution that has a precisely known concentration
how to prepare a standard solution
1. using a precise balance, carefully weigh out the required mass of solid onto a watch glass
2. transfer this solid to a clean, dry beaker and use some water to wash any bits of solid from the glass watch into the beaker
3. add water to the beaker to completely dissolve to completely dissolve the solid and use a glass rod to stir the solution to help the solid dissolve
4. once the solid has dissolved, transfer the solution into a volumetric flask. (use a volumetric flask that's the same size as the vol of solution you want to make up) rinse the beaker and glass rod with water, transferring this water into the volumetric flask
5. use water to fill the volumetric flask up to the graduation line. use a pipette to add the final few drops to make sure you don't add too much water and overshoot the gline
6. insert the stopper and invert several times to mix thoroughly
what equation do you use to calculate concentrations from standard solutions
moles = conc x volume in cm3(/100)
vol needs to be in DM3
how to calculate how much distilled water is needed to dilute to make further standard solutions of different concs
- divide the conc of the standard solution you want by the conc of the standard solution you have
- multiply by the volume of new standard solution you want = vol of concentrated solution to use
- subtract this volume from the total volume of dilute standard solution you desire = amount of distilled water to use
know how to do titration calculations.
use table and balance
what are oxidation numbers/states
of an element tells you the total number of electrons it has donated or accepted to form an ion or part of a compound
oxidation number of ELEMENTAL substances
e.g Mg, H2, P4
ZERO
oxidation number of SIMPLE monatomic IONS
e.g NA+, Mg2+
its CHARGE
Na+ = +1
Mg2+ = +2
oxidation number NEUTRAL COMPOUNDS
e.g MgCl2
ZERO
Mg = +2
Cl = -1 (x 2) = -2
-2 + 2 = 0
oxidation number of MOLECULAR IONS
e.g SO4^2- ion
SUM of all the elements o numbers which is equal to the IONS OVERALL CHARGE
O = -2 (x 4) = -8
S = +6 because overall charge (-2)
so -8 + 6 = -2
the oxidation number of oxygen is always __ EXCEPT...
-2
in peroxides (O2^2-), where it is -2
molecular oxygen (O2), where it is 0
systematic names (year 13?)
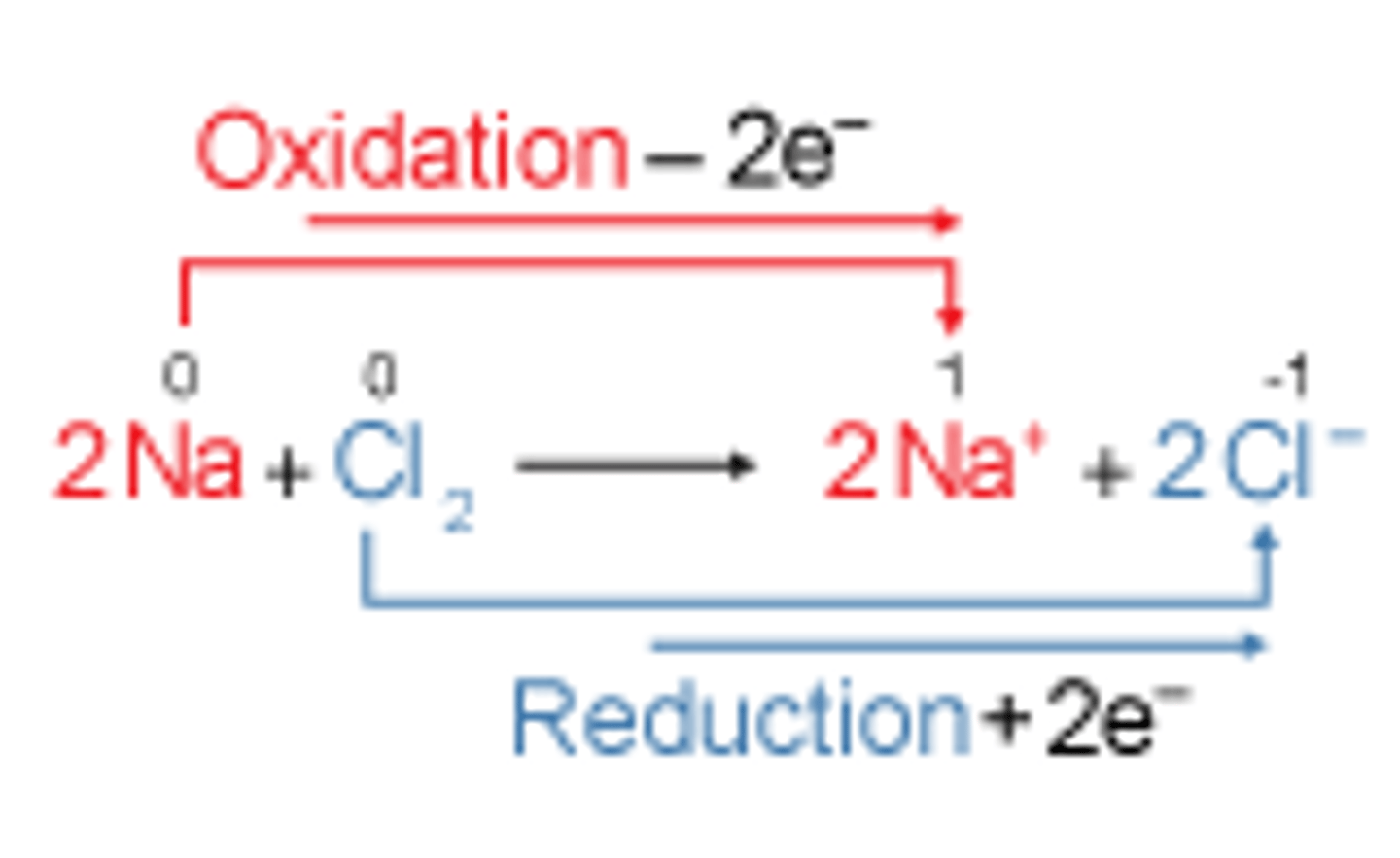
oxidation
gain of oxygen
loss of electrons
reduction
gain of electrons
loss of oxygen
what is a redox reaction
a chemical reaction in which both oxidation and reduction happen simultaneously, there is a transfer of electrons
what is an oxidising agent
accepts (gains) electrons and gets REDUCED
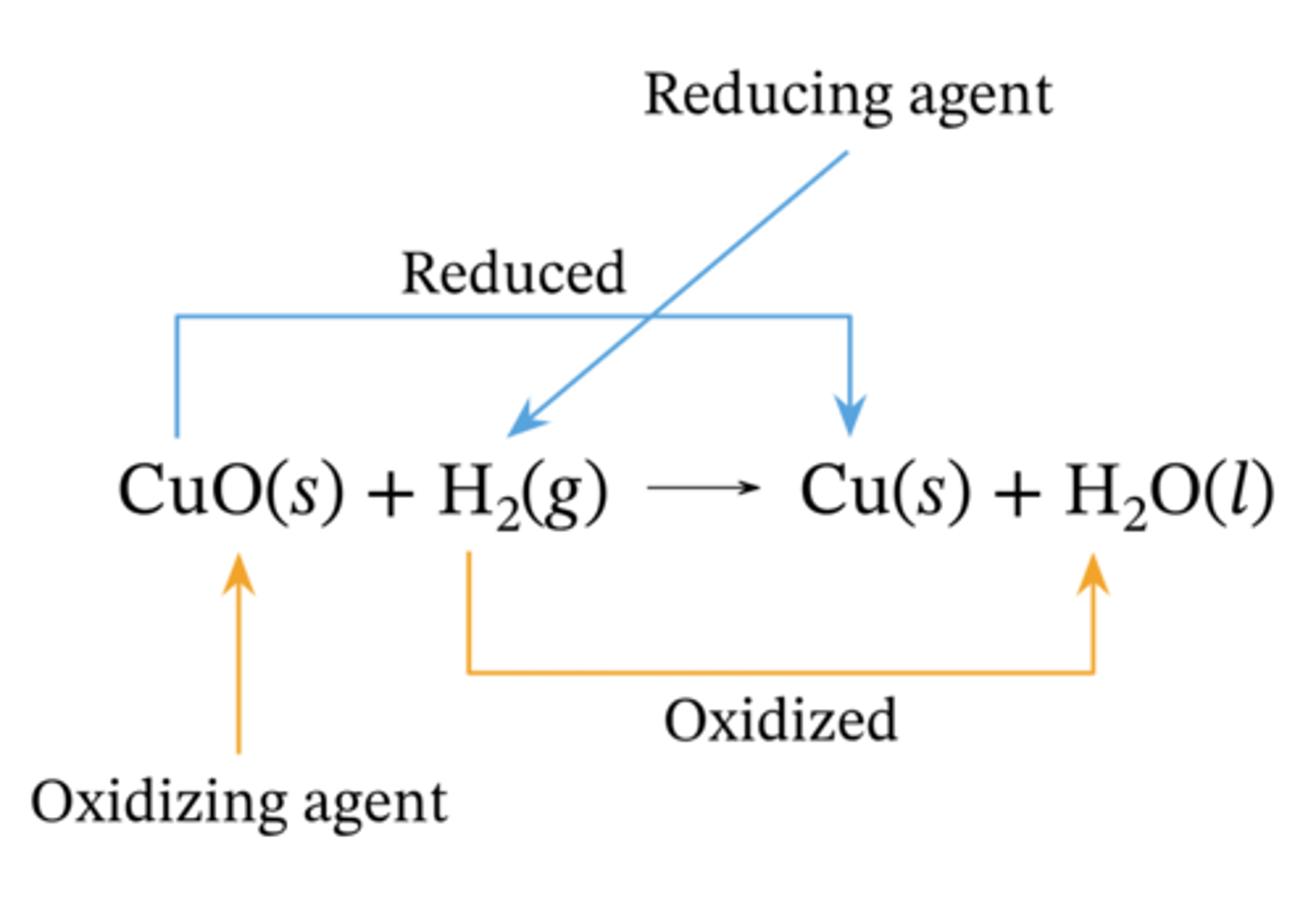
what is a reducing agent
donates (loses) electrons and gets OXIDISED
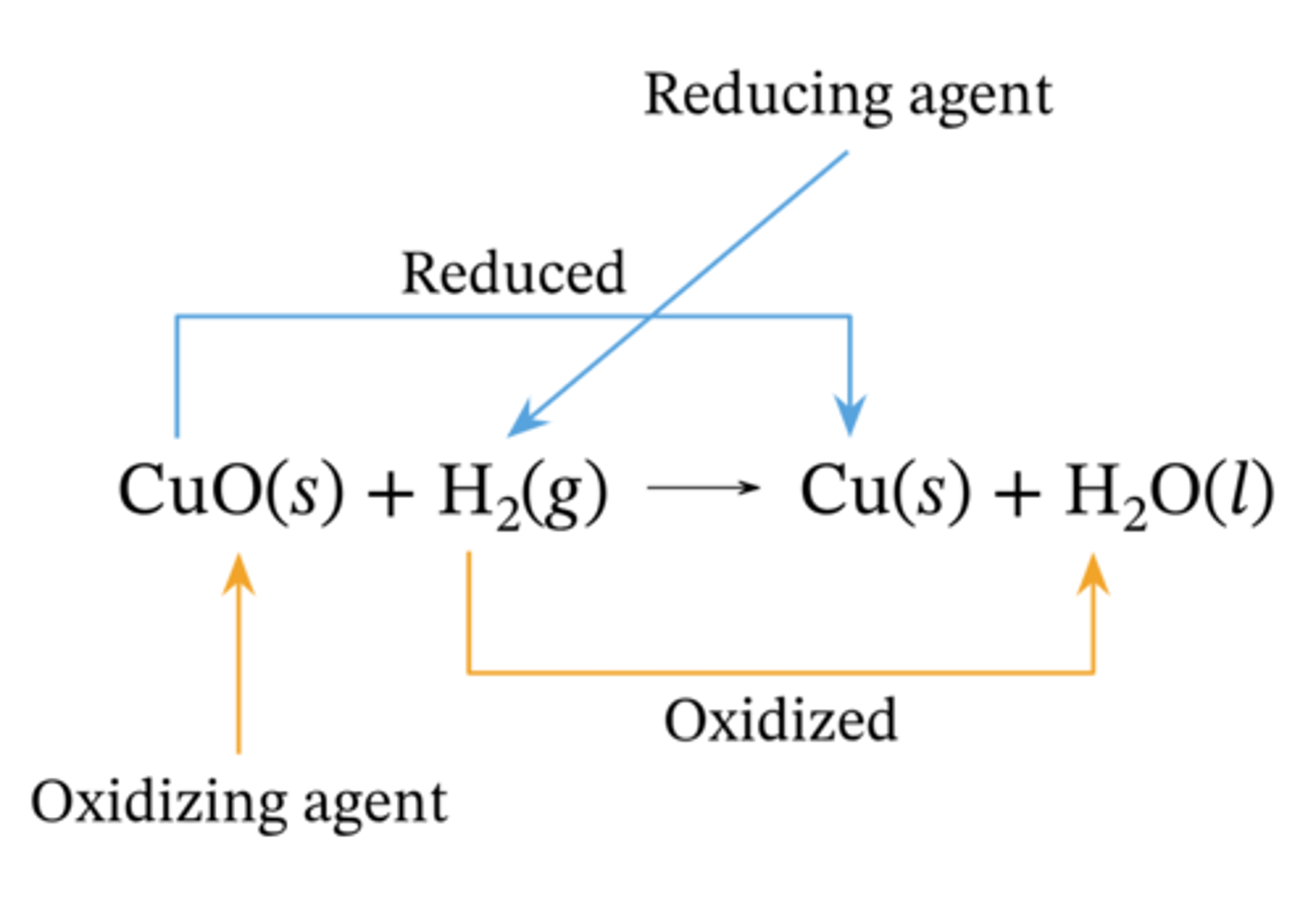
as an electron is lost (oxidised)
the oxidation number for an atom will INCREASE by 1 for every e- lost
as an electron is gained (reduced)
the oxidation number will DECREASE by 1 for every e- gained