Electronic Configuration
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
Blocks
The periodic table is split into four main blocks depending on electronic configuration. S block, p black, d block and f block
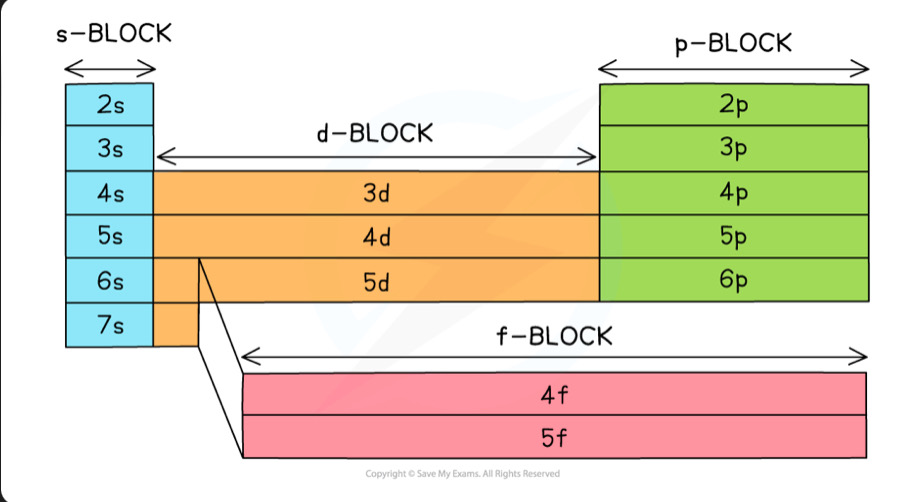
Sub shells
Increase in energy from s,p,d,f. The only exception is that 3d has a slightly higher energy than the 4s orbital
What does electronic configuration represent
The number of electrons in each shell, sub shell and orbital of an atom
1s1
The big one represents the shell number, “s” represents subshell and the small one represents the number of electrons
Principal energy level one
Subshell - 1s and maximum number of electrons is 2
Principal energy level two
Subshells - 2s,2p. Maximum number of electrons is 8
Principal energy level three
Subshells - 3s,3p,3d. Maximum number of electrons is 18
Principal energy level four
Subshells - 4s,4p,4d,4f. Maximum number of electrons is 32
Ions
The transition metals fill the 4s sub shell before the 3d sub shell but lose electrons from the 4s first and not the 3D, the 4s sub shell is lower in energy
Helium [He]
1s2 - the first 2 electrons
Neon [Ne]
1s2, 2s2, 2p6 - first 10 electrons
Argon [Ar]
1s2, 2s2, 2p6, 3s2, 3p6 - first 18 electrons