Chemistry 1.4- Energetics
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
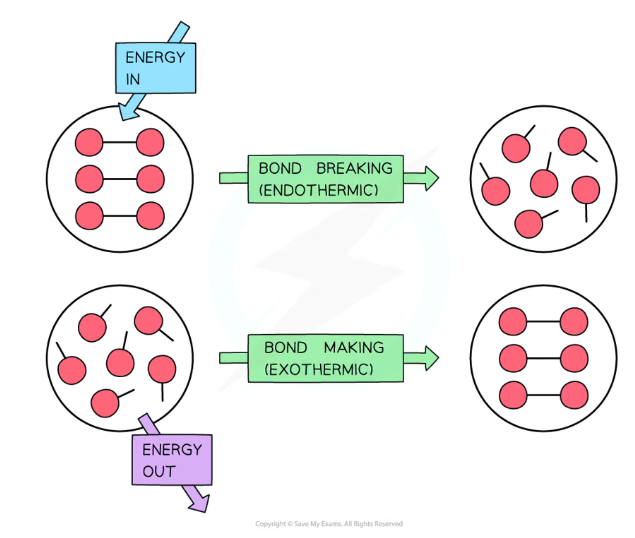
Bond breaking is…
Bond making is…
(Endo/exothermic)
Bond breaking is endothermic, it takes in energy
Bond making is exothermic, it releases energy
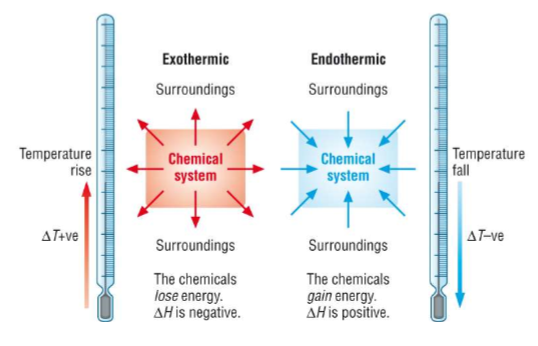
What is an enthalpy change? When is it positive and negative?
Enthalpy change (ΔH) is the heat energy change measured under constant pressure, which reflects the change in chemical energy
Exothermic reactions have a negative ΔH because energy is lost to the surroundings, so the products have less chemical energy stored
Endothermic reactions have a positive ΔH because energy is taken in from the surroundings, so the products have more chemical energy stored
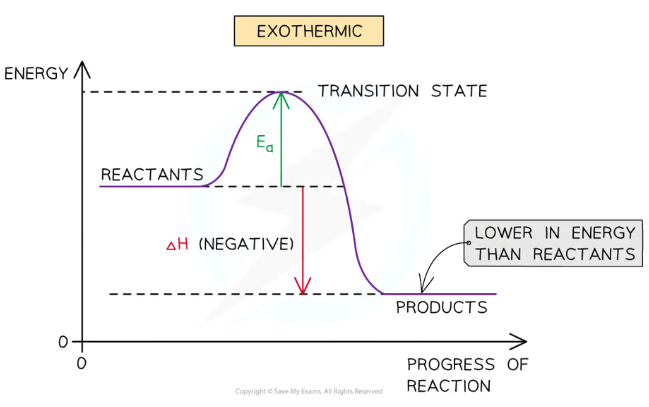
Exothermic energy level diagram
Products have less energy stored than the reactants, because that energy has been lost to the surroundings, so the enthalpy change (∆H) is negative
Requires less activation energy (Ea) than endothermic reactions
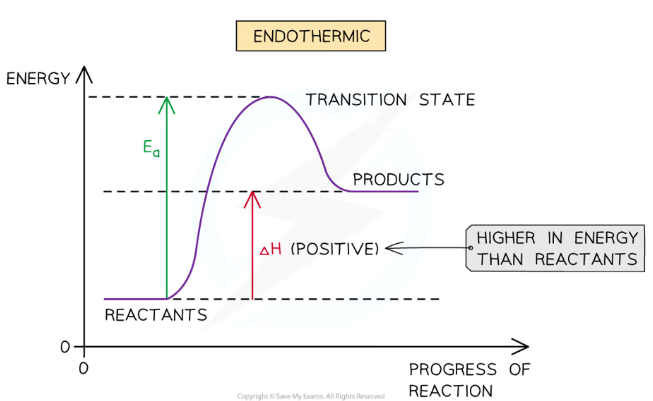
Endothermic energy level diagram
Products have more energy stored than the reactants, because energy has been taken in from the surroundings, so the enthalpy change (∆H) is positive
Requires more activation energy (Ea) than exothermic reactions
What are standard conditions for standard enthalpy changes?
A pressure of 100 kPa
A temperature of 298 K (25 oC)
Each substance is in its standard physical state (eg. oxygen is a gas, water is a liquid)
What is the symbol for standard enthalpy change?
ΔHꝊ
What is the standard enthalpy change of combustion?
The enthalpy change when one mole of a substance is burnt in excess oxygen (complete combustion) in standard states
ΔHcꝋ
Eg. C2H6 (g) + 3½O2 (g) → 2CO2 (g) + 3H2O (I) (one mole of ethane is burned)
What is the standard enthalpy change of formation?
The enthalpy change when one mole of a compound is formed from its elements under standard conditions
ΔHfꝋ
Eg. H2 (g) + ½O2 (g) → H2O (I) (one mole of water formed)
What is the standard enthalpy change of reaction?
The enthalpy change when a reaction occurs in the stoichiometric (simplest) equation under standard conditions
ΔHrꝋ
Eg. MgCO3 (s) → MgO (s) + CO2 (g) (all in simplest ratio)
What is the standard enthalpy change of neutralisation?
The enthalpy change when one mole of water is formed in the reaction between an acid and alkali under standard conditions
ΔHneutꝋ
Eg. ½H2SO4 (aq) + NaOH (aq) → ½Na2SO4 (aq) + H2O (I) (one mole of water formed)
What is specific heat capacity?
The energy needed to increase the temperature of 1g of a substance by 1oC
What is the formula for the enthalpy change of a reaction?
q=mcΔT
enthalpy change = mass x specific heat capacity x change in temperatre
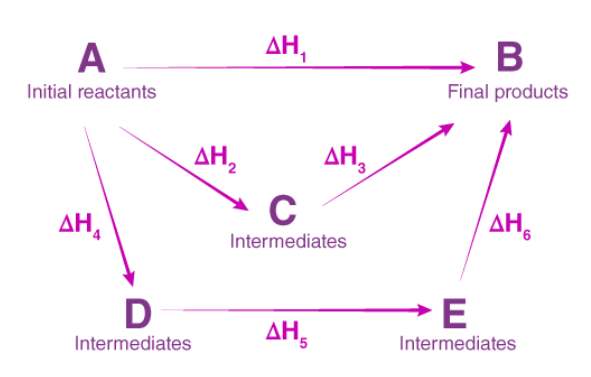
What is Hess’s law?
The enthalpy change of a reactions is the same regardless of the route the reaction takes
ie. The enthalpy change for the reaction (A → B), ΔHr is the same as the sum of the intermediate reaction enthalpies, ΔH2 + ΔH3 (A → C → B) or ΔH4 + ΔH5 + ΔH6 (A → D → E → B)
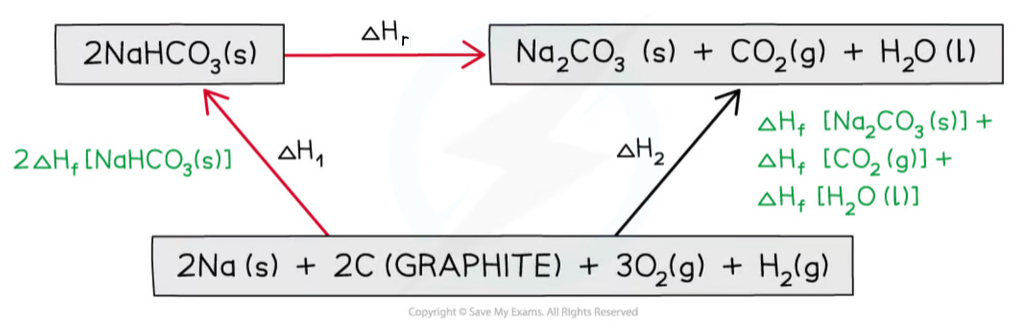
How do you calculate the enthalpy of reaction using Hess’s law?
ΔHr = ΔH2 (formation of products) - ΔH1 (formation of reactants)
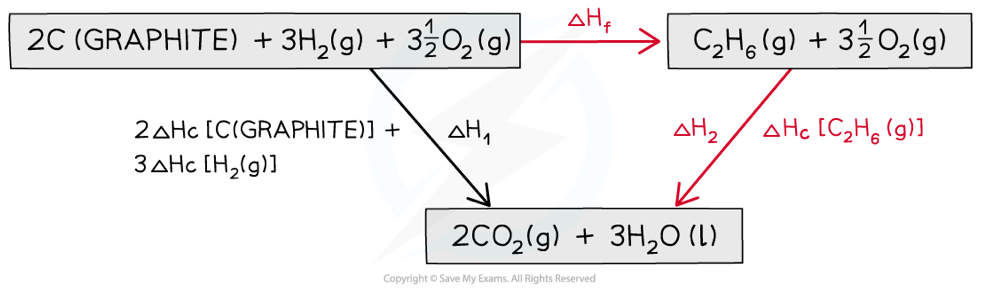
How do you calculate the enthalpy of formation using Hess’s law?
ΔHf = ΔH1 (combustion of reactants) - ΔH2 (combustion of products)
What is mean bond enthalpy?
The enthalpy required to break a covalent bond, averaged over the substances in which the bond is found
These are always given as positive enthalpies, as energy must be put into a system to break a bond
How can we use bond enthalpies to calculate the enthalpy of a reaction?
Enthalpy to break the bonds in the reactants - enthalpy released when making the bonds in the products
Why are Hess’s law calculations more accurate than bond enthalpy calculations?
Bond enthalpy calculations use mean values for each bond, averaged across each of its compounds, so it may use values that are inaccurate for the bonds in the specific compounds used in a reaction