Chemistry 2 CPE - FINAL EXAM STUDY GUIDE - DeMartini
1/61
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
62 Terms
Atoms
Consists of energy levels, sublevels, and orbitals where electrons are found.
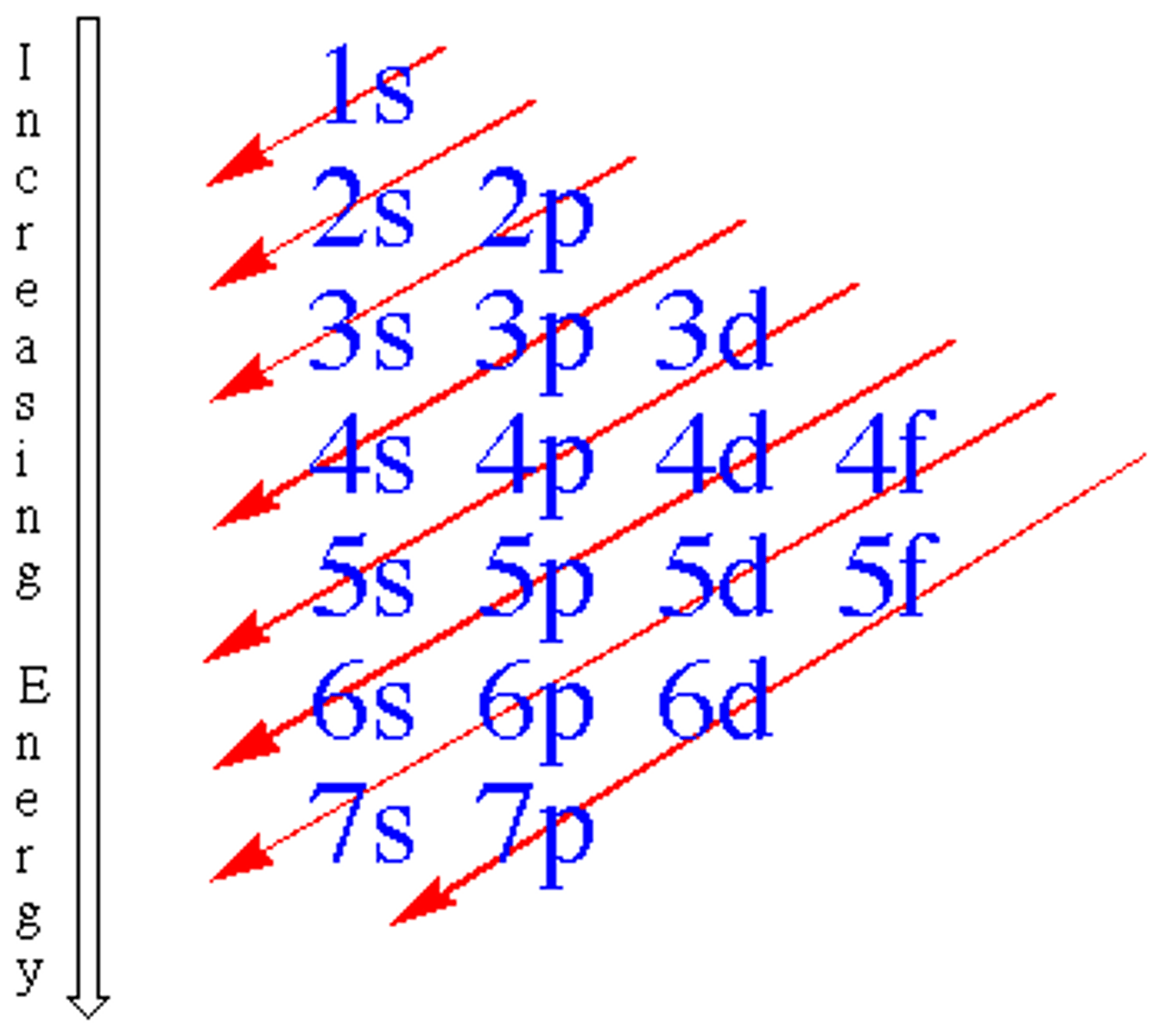
Electron configuration
The arrangement of electrons in an atom's energy levels and sublevels.
s
2
p
6
d
10
f
14
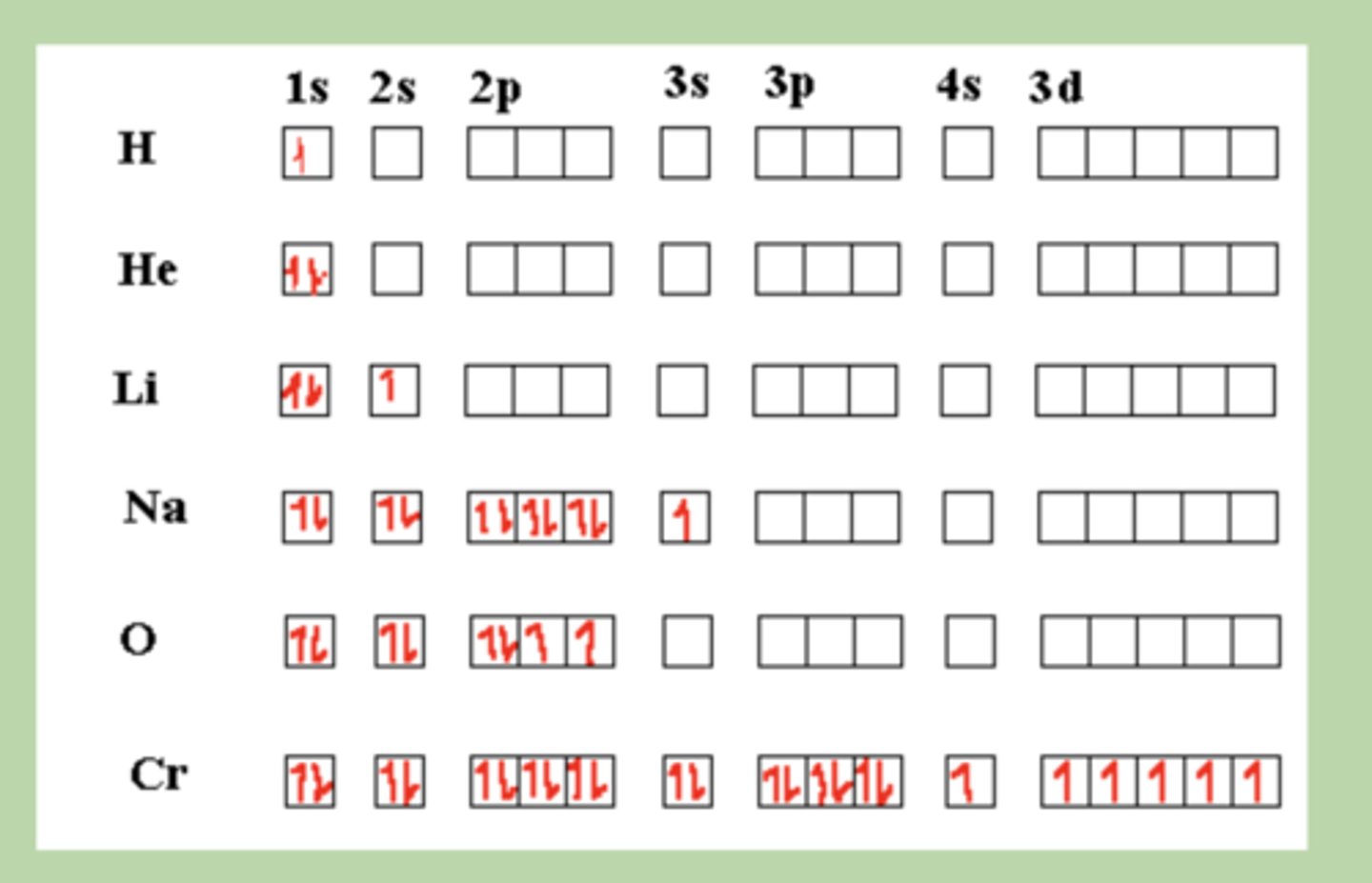
Orbital configuration
Shows electrons as arrows in boxes representing orbitals within sublevels.
Valence electrons
Electrons in the outermost energy level involved in bonding.
History of the periodic table
Created by Dimitri Mendeleev, who organized elements by increasing atomic mass and similar properties.
Periods 1-7
Horizontal rows on the periodic table, which indicate energy levels of electrons.
S~block
Left side
P~Block
Right side
D~block
Middle transition metals
F~block
Bottom inner transition metals
Group 1 – Alkali Metals
1 valence electron
VERY REACTIVE
Not typically found in nature as free elements because they are so reactive.
Combine with non-metals and react strongly with water to produce hydrogen gas and aqueous solutions
Group 2 – Alkaline Earth Metals
2 valence electrons
Harder, denser, and stronger than alkali metals.
Higher melting points
Too reactive to be found in nature as free elements.
Transition Elements.Gr 3-12 “d block”
Some element electron configurations are not consistent with the orderly d sublevel filling order
Metals with metallic properties
relatively unreactive
Noble Gases
Group 18
helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn), and oganesson (Og).
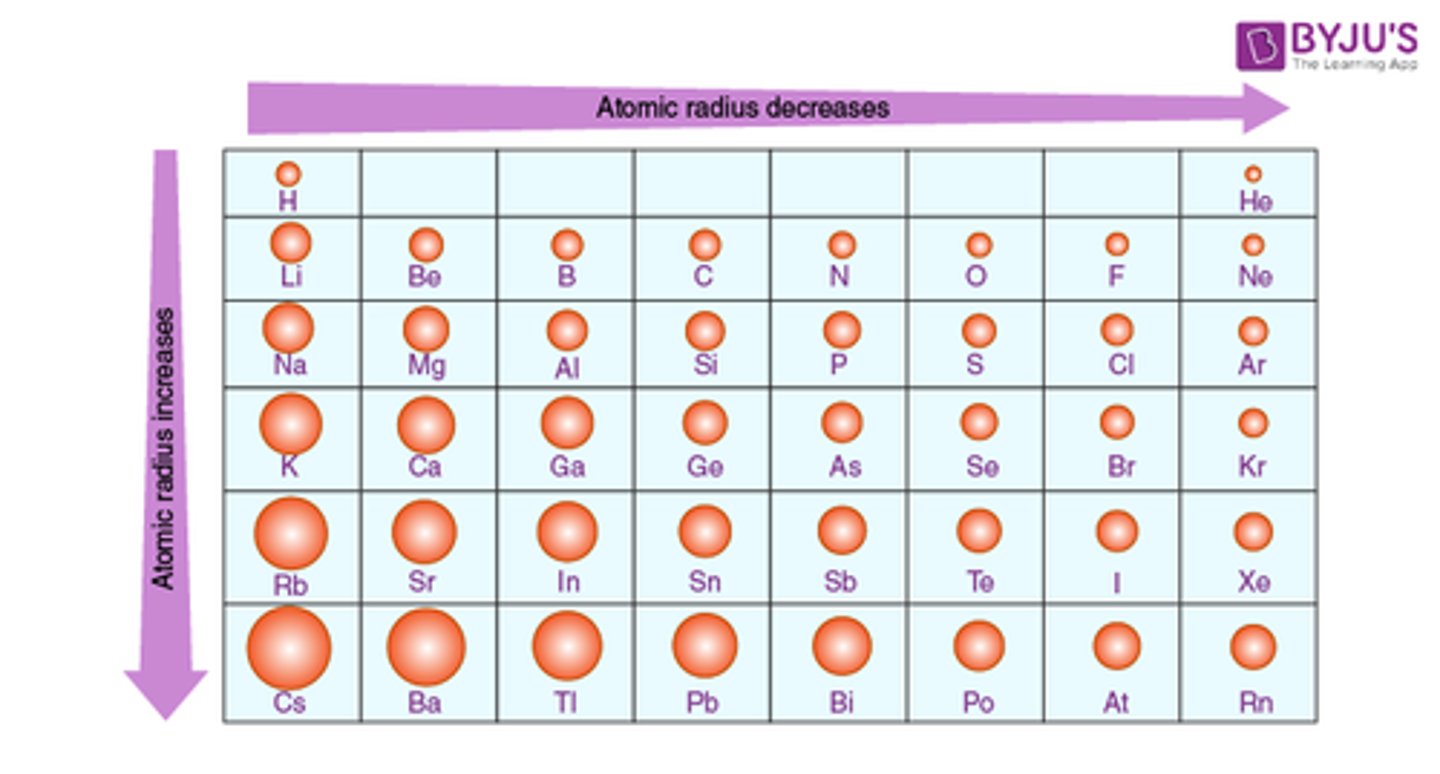
Atomic radius
Size of an atom, Decreases across a period and Increases down a group.
Halogens
Consists of elements in group 17 on the Periodic Table (F, Cl, Br, I, At)
Highly reactive nonmetals
7 valence electrons
Ionic radii
The size of an ion: cations are smaller, and anions are bigger.
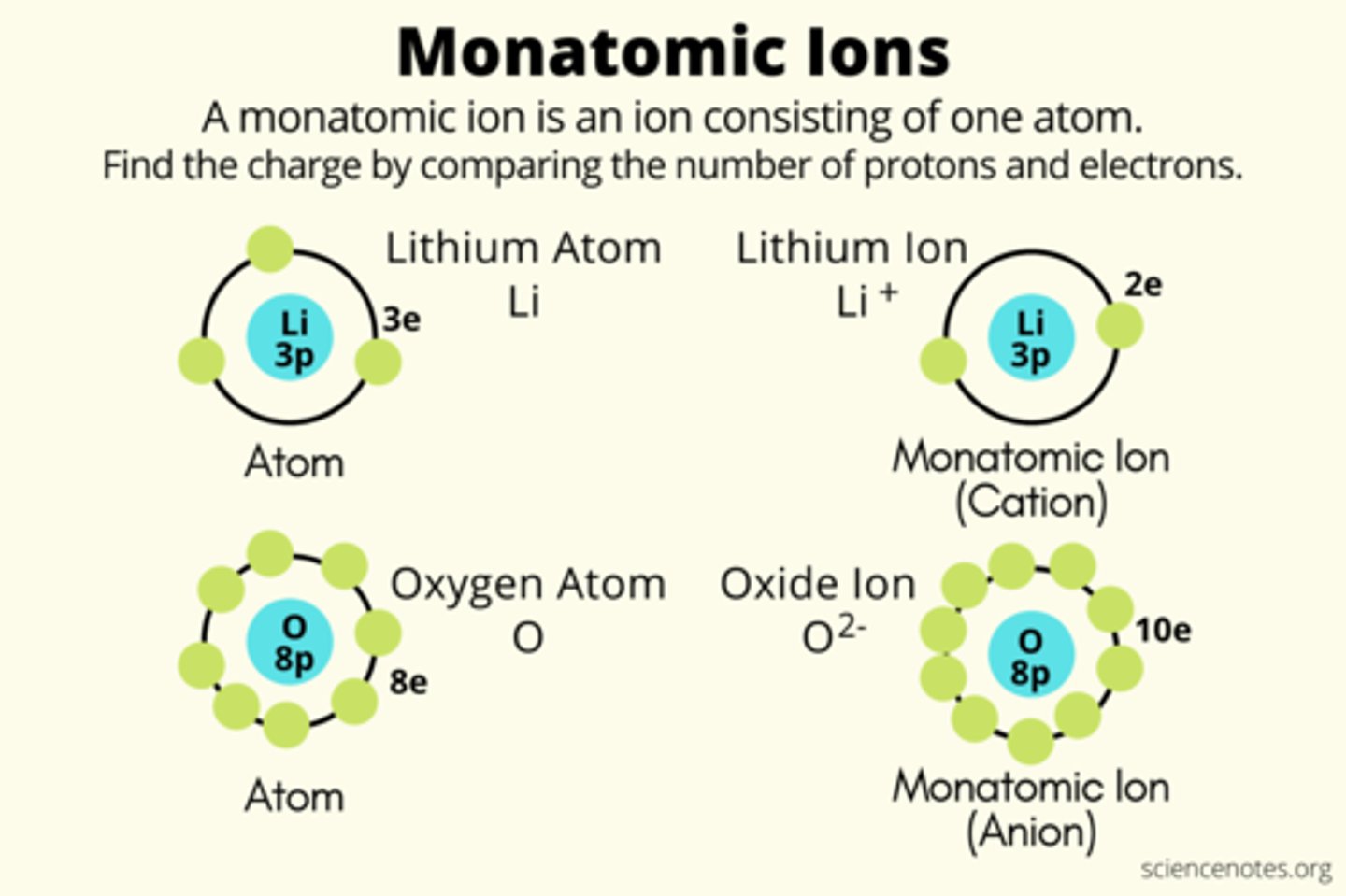
Cations
Positively charged ions
Anions
Negatively charged ions
Protons
Positively charged particles
Electrons
Negatively charged particles
Neutrons
Neutral charge
Ionization energy
Energy required to remove an electron from an atom, increases across a period, decreases down a group.
Ionization energy generally increases across a period (left to right) due to increasing nuclear charge, while it decreases down a group (top to bottom) due to increasing atomic radius and electron shielding
Electron affinity
Energy change when an atom gains an electron, generally becomes more negative across a period.
When an atom gains an electron, it becomes a....
Negatively charged ion called an anion. This is because the atom now has more electrons than protons, resulting in a negative charge.
Electronegativity
Ability of an atom to attract electrons in a bond, increases across a period, decreases down a group.
Electronegativity decreases as you move down a group in the periodic table because the atomic radius increases, making the nucleus less effective in attracting electrons. Conversely, electronegativity increases as you move across a period because the atomic radius decreases due to increasing nuclear charge.
Significance of a chemical formula
Shows the types and numbers of atoms in a compound.
Monatomic ions
Consist of a single atom with a positive or negative charge resulting from the loss or gain of one or more valence electrons
Binary ionic compounds
Involve combining the compound's (+) and (-) ions.
Cation is named first, and Anion follows (~ide) ending.
Stock system of naming compounds
Uses Roman numerals to show metal ion charge.
Type 2 Binary Ionic Compounds
These form between ONE metal from the d~block (groups 3-12) that forms more than one ion. You must use the stock system to name these compounds.
Naming compounds using prefixes
Used for covalent compounds to indicate the number of atoms. BOTH ELEMENTS MUST BE ABOVE THE STAIRCASE.
Naming ternary compounds
Compounds with three or more elements, often containing polyatomic ions.
Covalent vs. ionic bonding
Covalent bonds share electrons; ionic bonds transfer electrons.
Percent composition
Percentage by mass of each element in a compound.
How to find percent compositon
1) Find the molar mass of each element in the compound
2)Add up both molar masses of each element in the compound
3)Divide the mass of each element (calculated in step 1) by the molar mass of the compound (calculated in step 2).
Multiply the result by 100% to express it as a percentage.
Example:
Let's calculate the percent composition of carbon dioxide (CO2):
Carbon: Molar mass of C = 12.01 g/mol. In CO2, there is 1 carbon atom, so the mass of C in CO2 is 12.01 g/mol.
Oxygen: Molar mass of O = 16.00 g/mol. In CO2, there are 2 oxygen atoms, so the total mass of O in CO2 is 2 * 16.00 = 32.00 g/mol.
Molar mass of CO2: 12.01 + 32.00 = 44.01 g/mol.
Now, calculate the percent composition:
% Carbon: (12.01 g/mol / 44.01 g/mol) * 100% = 27.29%.
% Oxygen: (32.00 g/mol / 44.01 g/mol) * 100% = 72.71%.
You can verify that the percentages add up to 100%.
Empirical vs. Molecular formulas
Empirical is the simplest ratio; molecular is the actual number of atoms.
How to find empirical formula
Percent to mass, mass to moles, divide by small, multiply until whole.
How to find molecular formula
Find molar mass, divide by the given mass, and multiply by the empirical.
Indications of a chemical reaction
Formation of precipitates, gas, color change, temperature change.
(s)
solid
(l)
liquid
(g)
gas
(aq)
aqueous,
→
yields
Δ
heat added, catalysts indicated.
Coefficients and subscripts
Coefficients show the number of molecules(big number); subscripts show a number of atoms in a molecule.(small number)
Word equations vs. formula equations
Word equations use names, formula equations use chemical symbols and formulas.
Balancing equations
Adjust coefficients to have equal atoms on both sides.
Types of chemical reactions
Synthesis (combine), decomposition (break apart), single replacement, double replacement, combustion (react with O₂).
Mg + O2 → MgO2
Synthesis
2Al2O3 → 4Al + 3O2
Decomposition
Zn + 2HCl → ZnCl2 + H2
Single Displacement
2NaOH + CuSO4 → Na2SO4 + Cu(OH)2
Double Displacement
CH4 + 2O2 → CO2 + H2O
Combustion
Types of stoichiometry problems
Convert mole~to~mole, mole~to~mass, mass~to~mole, and mass~to~mass.
Percent yield
Actual yield divided by theoretical yield times 100%.
Limiting reactants
The reactant that runs out first, limiting the amount of product formed.