Physical Science Laws
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
Boyle’s Law Equation
P1 V1 = P2 V2
Charles’ Law Equation
V1/T1 = V2/T2
Two variables for Charles’ Law
Volume & Temperature
Two variables for Boyle’s Law
Pressure & Volume
Volume
The amount of space an object occupies.
Temperature
The amount of the kenetic energy of the particles.
Pressure
Caused by the particles colliding with each other & the walls of the/a container they are in.
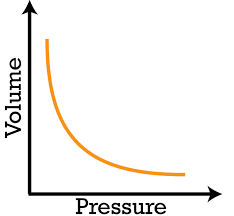
What type of graph is Boyle’s Law?
A Inverse Variation
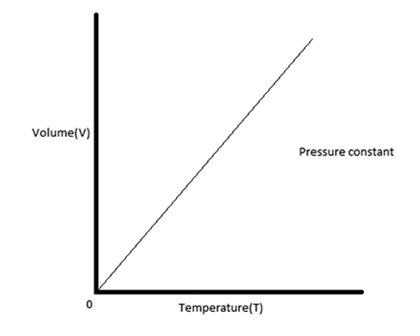
What type of graph is Charles’ Law?
A Directly Proportional
Boyle’s Law
For a fixed mass of gas at a constant temperature, pressure & volume are inversely proportional.
Charles’ Law
The volume of a gas is directly proportional to its absolute temperature when pressure is held constant.
Charles’ Law Graph
Boyle’s Law Graph