molecular and neural basis of circadian oscillations
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
what are circadian rhythms?
24 biological rhythms controlled by an internal clock
important for the sleep-wake cycle, metabolism (e.g insulin sensitivity), gut microbiota, control of cell division and apoptosis, aging and cardiovascular function
what are clock genes?
genes that produce proteins which generate and regulate circadian rhythms
what four clock genes form the primary transcriptional-translational feedback loop?
BMAL1 (activator)
CLOCK (activator)
CRY (repressor)
PER (repressor)
what is the transcription-translation feedback loop?
self regulating molecular circuit in which specific genes are transcribed into mRNA and translated into proteins which then feedback to inhibit their own genes transcription across a 24 hour period
this forms the basis of the circadian clock
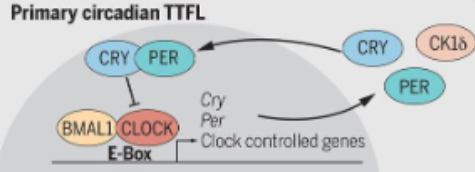
how does the primary transcription-translation feedback loop work?
in daytime, CLOCK and BMAL1 form a heterodimer in the nucleus and this complex binds to e-box elements in the promoter region of target genes
this activates transcription of these genes, producing PER and CRY mRNA
levels of PER and CRY in the cytoplasm gradually rise in the day
in the evening, PER-CRY complexes are formed in the cytoplasm and are stabilised by phosphorylation (by caesin kinase 1 epsilon/delta)
once levels of PER-CRY are high enough, the complex moves back into the nucleus
at night, the PER-CRY complex binds to the CLOCK-BMAL1 heterodimer which blocks CLOCK-BMAL1 activity preventing further transcription of PER and CRY genes
therefore PER and CRY inhibit their own production (-ve feedbackk loop)
over the night, PER and CRY proteins are phosphorylated and targeted for degradation by the ubiquitin-proteasome system
as they degrade, inhibition on CLOCK-BMAL1 is lifited, allowed transcription to resume
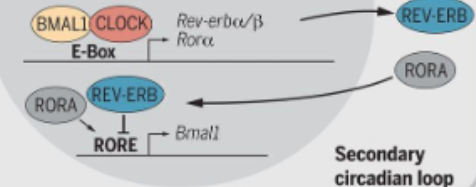
how does the secondary transcription-translation feedback loop work?
the CLOCK-BMAL1 complex activates the transcription of REV-ERBα/β and RORα/β/γ
both REV-ERBs and RORSs bind to the same DNA elements in the BMAL1 promoter- RORE sites- but have opposite effects
RORα/β/γ is an activator and increases BMAL1 transcription, REV-ERBα/β is a repressor and suppresses BMAL1 transcription
TF when REV-ERB levels drop, ROR dominates and this creates a rhythmic oscillation of BMAL1 expression
what two clock genes form the secondary transcriptional-translational feedback loop?
REV-ERBα/β
RORα/β/γ
what is the significance of the secondary TTFL?
stabalises the rhythm of the core clock
strengthens amplitude of oscillations- preventing flat rhythms
links metabolism and circadian control (e.g REV-ERBs and RORs involved in lipid metabolism and glucose homeostasis)
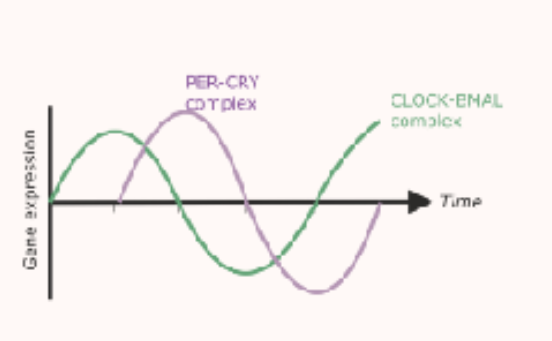
what type of relationship is formed between CLOCK-BMAL1 and PER-CRY?
a close to anti-phasic relationship
means that two rhythms peak at opposite times (when one is high one is low)
at daytime, CLOCK-BMAL1 activity is high and PER-CRY low
at nighttime, PER-CRY activity is high, CLOCK-BMAL1 is low
however it is not perfectly opposite as there is a bit of lag time due to the time it takes for the PER-CRY molecules to be made and accumulate in the nucleus
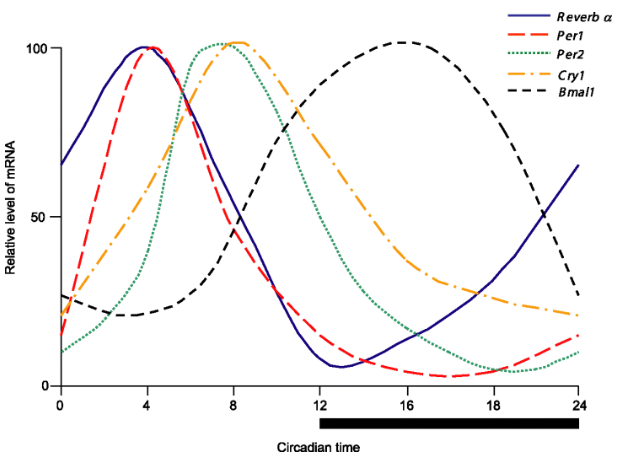
what type of relationship is formed between CLOCK-BMAL1, PER-CRY and REV-ERB & ROR?
morning = CLOCK-BMAL1 at peak
afternoon = REV-ERBα at peak, ROR outweighed by REV-ERB
night = PER-CRY and REV-ERBα high
late night/early morning = PER-CRY degrades and RORα rises
system then resets and the next wave begins
what is the link between molecular feedback and electrophysiological intergration?
inside the nucleus, the TFFL takes place
this influences intracellular pathways such as: calcium signalling, kinase activity and redox state (balance of NAD/NADH) which affect ion channel function
electrical properties of SCN neurons are therefore controlled by the molecular clock
ion channels are more excitable during the day and are hyperpolarised at night
this is a bidirectional relationship
how does gene expression drive membrane potential firing?
during the day, molecular clock output (CLOCK-BMAL1) enhances excitatory ion channel activity (Na+ and Ca2+), keeping the membrane depolarised (up-state reinforcement)
at night, the accumulation of inhibitory influences via PER-CRY, GABAergic signalling and K+ channel activation hyperpolarises rhe cell (down-state reinforcement)
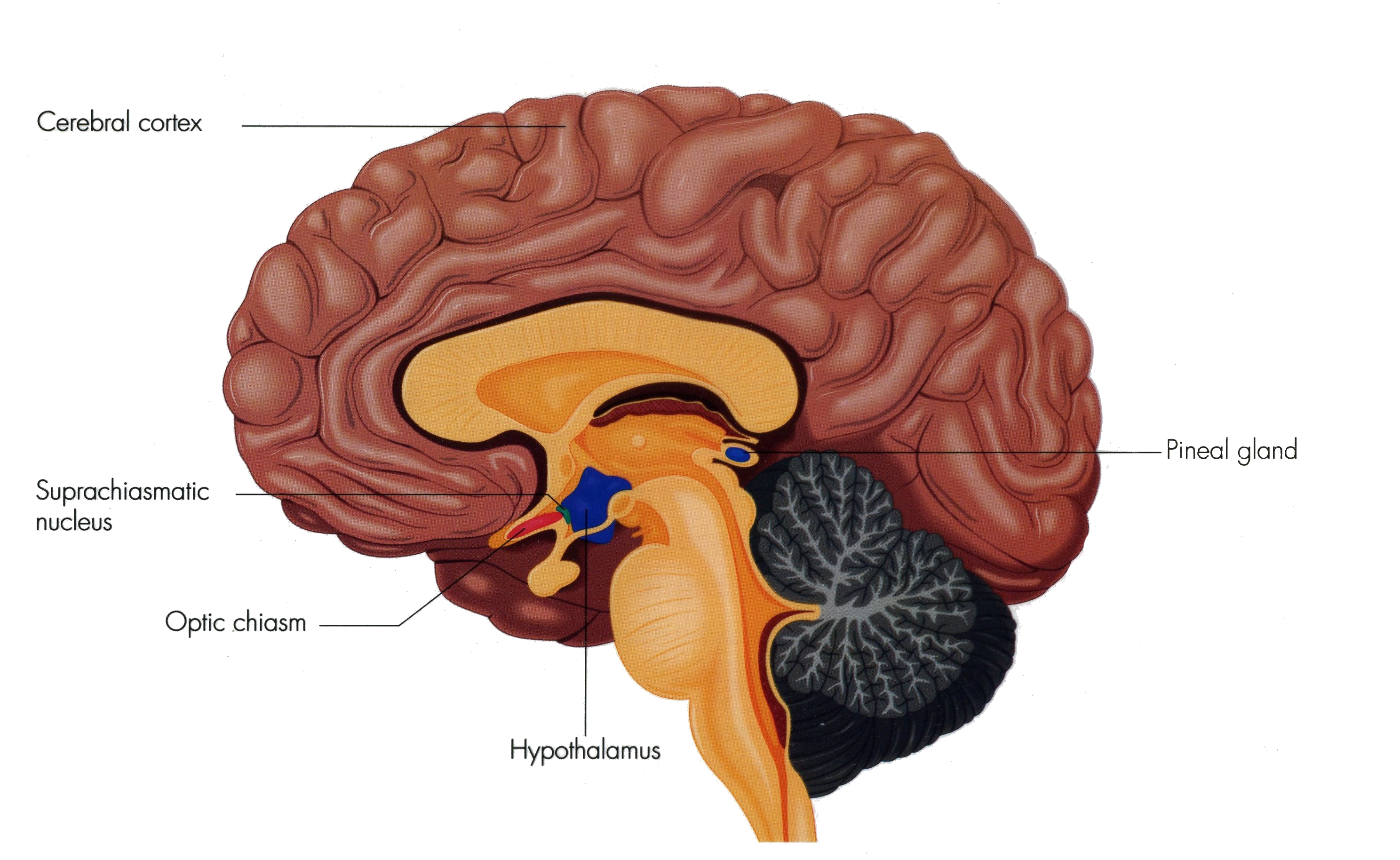
what is the suprachiasmatic nucleus?
master circadian clock of the brain
located in the hypothalamus and just above the optic chiasm
light sensitive cells in retina send signals dircetly to SCN via retinohypothalamic tract to entrain body to day/nigh
acts as the body’s master pacemaker
what do scn lesions in mice cause?
disrupted sleep wake cycle
disrupted locomotion
disrupted hormone secretion
disrupted metabolism
disrupted body temperature
disrupted cardiovascular physiology
what is an SCN graft?
a surgical implantation of suprachiasmatic nucleus tissue into the brai of an animal whose endogenous SCN has been removed or damage to restore circadian rhythms
can SCN lesion (SCN-x) be restored by foetal SCN grafts?
demonstarted by actogram
x axis = time of day (0-48 hours), y axis = consecutive days of recording
black marks = period of activity, white = inactivity
LD = light-dark cycle, DD = constant darkness
before grafting, actogram shows amimal is arrhythmic and after the SCN graft diagonal bands appear, showing the animal is active on a ~24 hour cycle again
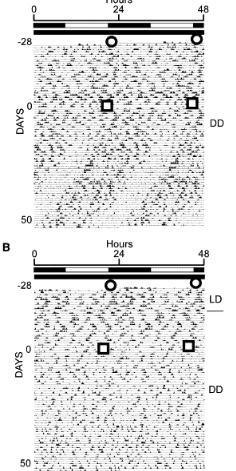
can SCN lesion (SCN-x) be restored by foetal cortex grafts?
no, animal reamins arrhythmic
this means only SCN tissue contains the necessary molecular machinery to generate circadian rhythms
what are CRY1 and CRY2?
part of the cryptochrome family
core transcriptional repressors that directly inhibit CLOCK-BMAL1
does deletion of CRY1 and CRY2 abolish the SCN clock?
single knockout:
CRY1 = clock still runs but shorter period (~22 hours)
CRY2 = clock still runs but longer period (~25 hours)
double knockout:
negative feedback can no longer function as PER alone cannot inhibit CLOCK-BMAL1,stopping the molecular oscillation of the SCN
how does light effect CRY1/2 knockout?
in LD conditions, CRY1/2 KO mice appear behaviourally rhythmic meaning that although their internal clock is broke, the mice still show daily activity rhythms
this is because this pattern is driven externally by light not internally by the SCNs clock = masking/light-driven behaviour
when you take an SCN slice from CRY1/2 mice and record neurnal firining, oscillations are very weak (small amplitude) or non-existent
TF the SCN clock (TTFL) requires CRY1 and CRY2 for strong, self-sustaining rhythmicity — but light can still impose apparent behavioral rhythms externally
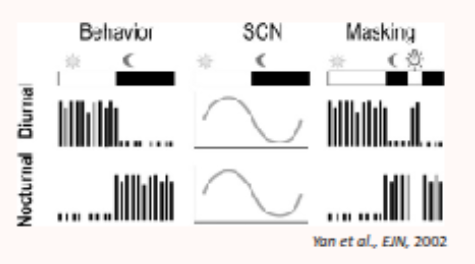
is there a difference in SCN activity between nocturnal and diurnal amimals?
no, in both nocturnal and diurnal species, SCN neurons are most active in the light phase and leas active in the dark phase (day-active structure across all animals)
even though SCN activity is the same, the behavioural output is the opposite
e.g nocturnal animals have high SCN firing in the light but behvaiour is active during the day TF the SCN inhibits the activity during the day
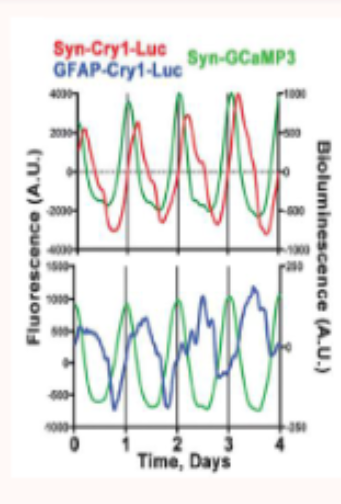
how do astrocytes in the SCN influence neuronal rhythms?
red line = CRY1 in neurons
blue line = CRY1 in astrocytes
green line = neuronal calcium activity
this shows that both neurons and astrocytes display seld sustained circadian oscillations and these ehythms are anti-phasic, therefore astrocytes and neurons oscillate out of phase but are functionally couples
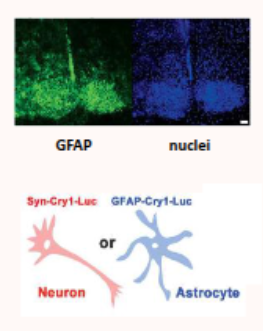
do astrocytes have clock genes?
GFAP is a marker for astrocytes
nuclei marks all cells
this shows the distribution of astrocytes within the SCN
also shows that CRY-1 is expressed in astrocytes
what is the contribution of astrocytes to SCN timekeeping?
astrocytes in the SCN display circadian oscillations of intracellular calcium which is high at night
extracellular glutamate release is in phase with astrocytic calcium rhythms and anti-phase to neuronal firing
this modulates neuronal excitability
clock gene rhythms also drive oscillations
TF rhythmically express clock genes what results in their calcium activity
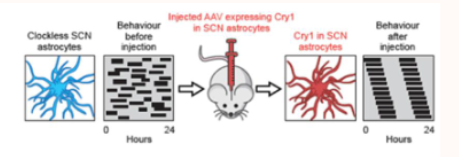
can astrocytes alone regulate the SCN?
if you give the CRY1 back to only astrocytes in the SCN (not neurons) then the animal will show normal circadian behaviour again
this means astrocytes alome can drive the master clock and are sufficient to impose 24-hour timing on neurons
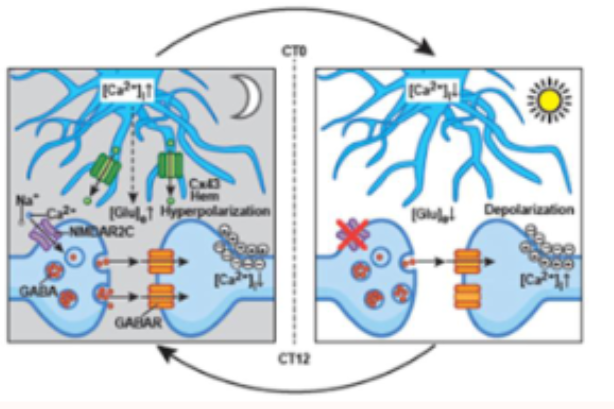
how do astrocytes drive timekeeping?
at night:
astrocytic intracellular calcium us high
astrocytes release ore extracellular glutamate
this activates presynaptic NMDA receptors (NMDAR2C)
this increases GABA release = neurons fire less
in day:
clearance of extracellular glutamate by reduced astrocytic glutamate release
relieves GABAergic tone
leads to depolarisation and increased electrical firing of SCN neurons
what is the uniclock model?
a conceptual framework proposed to explain how the SCN maintains circadian rhythms when only one type of cellular clock is present (neurons OR astrocytes)
what is the multiclock systen?
the body contains many circadian clocks, not just a single one in the SCN e.g peripheral clocks in almost evert organ and skin type
each local clock has its own molecular machinery and generates its own 24 hour rhythm
what is the evidence for the multiclock system?
genetically engineered mouse that expresses luciferase fused to PER2 (mPer2Luc knock in)
when PER2 is activate, luciferase produces light
therefore light output = PER2 expression = molecular clock rhythm
work in different tissues demonstrates bioluminescence in SCN, cortex, liver, lungs etc
however some peripheral clocks dampen over time
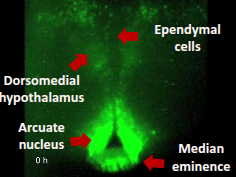
what are the extra-SCN ocscillations of the hypothalamus?
neuronal and non-neuronal rhythms are found in the medio-basal hypothalamus
first single-cell recordings of circadian oscillations elsewhere in the brain
HE less robustness, amplitude and synchrony in contrast to the SCN
what are the extra-SCN ocscillations of the brainstem?
neuronal and non-neuronal rhythms present in the dorsal vagal complex
ependymal (non-neuronal) are in antiphase to neuronal rhythms
DVC clock is metabolically entrainable: what animals eat (e.g high fat diet, calorie restriction) and when they eat
what are the extra-SCN ocscillations of the choroid plexus?
CP = produces CSF and made up of modified ependymal cells
ependymal cells express clock genes with robust circadian rhythms
HE this is not passive, send signals that affect the SCN
how does the chloroid plexus influence the SCN?
if you genetically manipulate the clock in the choroid plexus (e.g kockout or overexpression) then the SCN period changes
this means the SCN is not completely autonomous as it receives rhythmic feedback from the choroid plexus
likely through CSF composition changing across the day, secretion factors, interaction with SCN neurons/astrocytes via ventricular CSF batthing nearby tissue
how can the choroid plexus unidirectionally affect its timekeeping?
if you manipulate the clock in the chloroid plexus (e.g change PER2/BMAL1) then the SCNs circadian period changes
HE if you manipulate the SCN’s clock then the choroid plexus doesnt change