Practice questions on R1.1 Measuring energy changes
1/9
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
Which are correct statements?
I. Heat is a form of energy.
II. Temperature represents a measure of the average kinetic energy of particles in a system.
III. The energy of a system cannot change.
a. I, II and III
b. I and II only
c. I and III only
d. II and III only
b. I and II only
heat is a form of energy that is transferred between systems and surroundings and moves from hotter to colder surfaces.
temperature does represent a measure of the avg. kinetic energy of particles in a system.
→ not all particles have the same kinetic energy (kinetic energy distribution)
→ energy of a system can change as it can move from one system to another
e.g exothermic reaction moves from the system to its surroundings
What conditions are substances assumed to be under when values like standard enthalpy of formation are given?
a. pure substances in their standard states at 298 K
b. pure gaseous substances at 100 kPa and 298 K
c. pure gaseous substances at 273 K
d. pure substances in their standard states at 100 kPa and 298 K
d. pure substances in their standard states at 100 kPa and 298 K
even tho temperature is not included as part of the standard symbol ‘⦵’ - chemists tend to assume a temperature of 298 K (25 °C), so that should be included in any answer given.
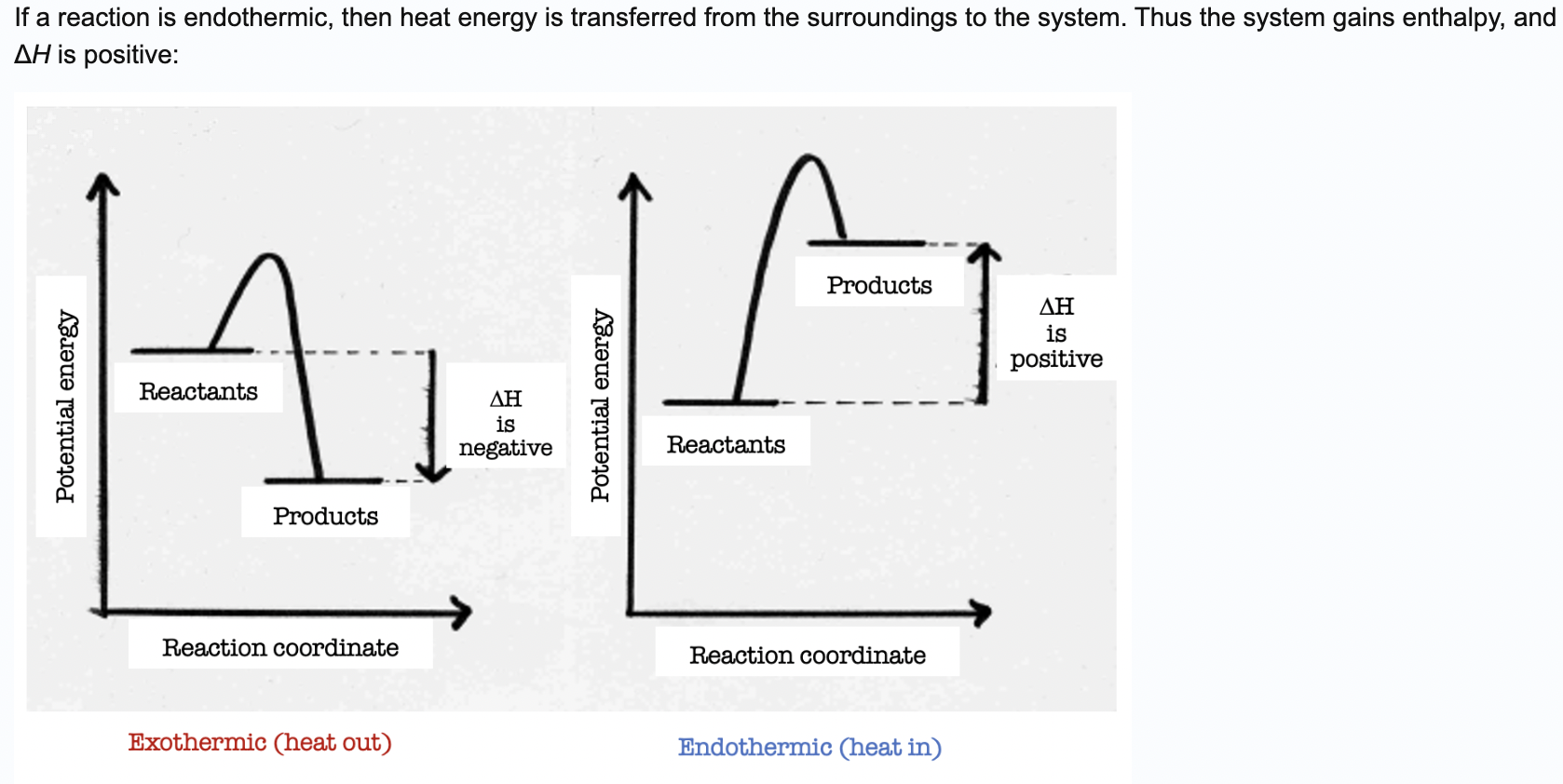
If a chemical reaction is endothermic in the forward direction, which of these statements is true?
a. The enthalpy of the products is higher than the enthalpy of the reactants and ΔH is negative.
b. The enthalpy of the products is lower than the enthalpy of the reactants and ΔH is negative.
c. The enthalpy of the products is lower than the enthalpy of the reactants and ΔH is positive.
d. The enthalpy of the products is higher than the enthalpy of the reactants and ΔH is positive.
d. The enthalpy of the products is higher than the enthalpy of the reactants and ΔH is positive.
A chemical reaction is carried out in aqueous solution and the temperature of the solution rises.
Which of these statements is true?
a. The enthalpy of the products is higher than the enthalpy of the reactants.
b. The bonds in the products are stronger than the bonds in the reactants.
c. ΔH is positive.
d. Heat energy is transferred from the surroundings to the system.
b. The bonds in the products are stronger than the bonds in the reactants.
when temperature of the solution increases, the system releases new energy in the surroundings. → exothermic: gives out heat
this means that bond forming (product bond; exo) is stronger since more energy is given out
Which equation represents the standard enthalpy change of formation, ΔHf⦵, of water?
a. 2H2(g) + O2(g) → 2H2O(l)
b. 2H2(g) + O2(g) → 2H2O(g)
c. H2(g) + ½O2(g) → H2O(g)
d. H2(g) + ½O2(g) → H2O(l)
d. H2(g) + ½O2(g) → H2O(l)
ΔHf⦵ = enthalpy change when 1 mole of a compound in its standard state formed from its constituent elements under their standard states (at 100 kPa and 298 K).
Answer (c.) is for gaseous state of water (not standard)
Answers (a.) and (b.) are for the formation of two moles of liquid water and gaseous water respectively.
Which equation represents the standard enthalpy change of combustion, ΔHc⦵, of propene, C3H6?
a. C3H6(g) + 3O2(g) → 3CO(g) + 3H2O(l)
b. C3H6(g) + 4½O2(g) → 3CO2(g) + 3H2O(l)
c. 2C3H6(g) + 9O2(g) → 6CO2(g) + 6H2O(l)
d. 2C3H6(g) + 9O2(g) → 6CO2(g) + 6H2O(g)
b. C3H6(g) + 4½O2(g) → 3CO2(g) + 3H2O(l)
ΔHc⦵ = the enthalpy change when 1 mole of a compound combusts completely (in excess oxygen) with all reactants and products in their standard states at 100 kPa and 298 K.
Complete combustion of hydrocarbons will always produce CO2+H2O
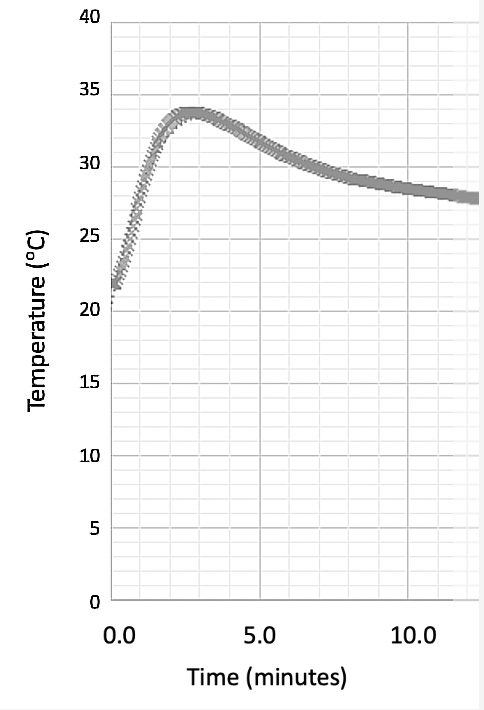
An experiment was carried out with a datalogger on an exothermic reaction, giving the following graph:
Given that the initial temperature was 21.5 ℃, what is the change in temperature, ΔT (to within ± 1 ℃), for the reaction?
a. 12.5
b. 15.0
c. 24.5
d. 36.5
b. 15.0
The graph should be extrapolated back to time = 0 to account for the heat lost to the surroundings.
This gives a value of 36.5 ℃, and a temperature change of 36.5 − 21.5 = 15.0 ℃.
A student investigated a combustion reaction using a spirit burner to burn some fuel and heat 200.0 cm3 of water. During the experiment, the water temperature increased from 22.0 °C to 38.4 °C.
How much heat energy, in kJ, was absorbed by the water?
a. 13.7
b. 13700
c. 22100
d. 22.0
a. 13.7
Using Q = mcΔT and a specific heat capacity of water of 4.18 J g−1 K−1 (both given in the IB data booklet):
Q (heat energy) = mass × specific heat capacity × temperature change (these three values are for whatever substance is absorbing the heat energy; in this case, water).
Water has a density of 1 g cm−3 (so 200 cm3 ≡ 200 g water).
38.4 − 22.0 = 16.4 °C (A temperature change of 16.4 °C is the same as 16.4 K).
Q = 200 × 4.18 × 16.4 = 13710.4 J = 13.7 kJ (3sf)
A student investigated the enthalpy change for solid ammonium chloride (NH4Cl) dissolving in distilled water.
The student added 5.59 g of ammonium chloride to 100 cm3 of water and measured a temperature decrease of 5.0 °C.
What is the enthalpy change for the dissolving process for one mole of ammonium chloride (in kJmol−1)?
a. +20
b. +20000
c. −20000
d. −20
Q = 100 × 4.18 × 5.0 = 2090 J (for 5.59g of NH4Cl)
Molar mass (NH4Cl) = 14.01 + (1.01× 4) + 35.45 = 53.50 g mol−1
Amount of NH4Cl used = mass ÷ molar mass = 5.59 ÷ 53.50 = 0.1045 mol
For one mole of ammonium chloride, the heat energy gained will therefore be:
2090 ÷ 0.1045 = 20000 J mol−1
≈20 kJ mol−1 (2sf)
The enthalpy change is endothermic because the temperature dropped; therefore ΔH is positive.
A student investigated a combustion reaction using a spirit burner to burn 0.400 mol of fuel and heat 200.0 cm3 of water in a 140.0 g copper calorimeter. During the experiment, the temperature of the water and the copper calorimeter increased from 21.4°C to 46.8°C.
The specific heat capacity of copper is 0.385Jg−1K−1, and of water is 4.18Jg−1K−1.
What is the enthalpy change of combustion for this fuel (in kJmol−1)?
a. −53.1
b. −56.5
c. −9.00
d. −90.3
b. −56.5
Temperature change: 46.8 − 21.4 = 25.4℃
For the water: Q = 200 × 4.18 × 25.4 = 21234.4J
And for the copper calorimeter:
Q = 140 × 0.385 × 25.4 = 1369.06J
Total Q = 21234.4 + 1369.06 = 22603.46J
→ for the 0.40 mol of fuel burnt
For one mole of fuel the heat energy transfer will therefore be:
22603.46 ÷ 0.40 = 56508.65Jmol−1
≈56.5 kJ mol−1 (3sf)
The enthalpy change is exothermic; therefore -ΔH.