Fundamental Biomolecules
1/34
Earn XP
Description and Tags
this is fucking chemistry??
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
35 Terms
What are the 6 essential elements that are used a lot in the human body?
C, H, O, N, P, S
What is significant of the element Iron?
carries oxygen from lungs to tissues and muscles via hemoglobin in red blood cells
What is significant of the element Potassium? (3)
-required for normal cell function
-maintains intracellular fluid volume
-blood pressure regulation
What is significant of the element Calcium? (2)
-maintain strong bones and teeth
-muscle contraction
What is significant of the element sodium? (4)
-maintains cellular homeostasis
-blood pressure regulation
-osmosis in extracellular fluid
-electrolyte balance
What is significant of the element zinc? (3)
-immune system function
-healing
-hormone production
What is significant of the element magnesium? (2)
-muscle and nerve function
-healthy immune system
What is significant of the element iodine? (2)
-thyroid hormones
-regulate metabolism
What is significant of the element lithium?
mood stabilizer
What is significant of the element chlorine? (2)
-digestion
-regulate blood pressure
What is significant of the element bromine?
tissue development
What is significant of the element fluoride? (2)
-prevents tooth decay
-stimulates new bone growth
What is significant of the element copper? (2)
-form red blood cells
-brain function
What is significant of the element cobalt? (2)
-cellular metabolism
-DNA repair and synthesis
What is significant of the element nickel? (3)
-iron absorption
-glucose metabolism
-cell growth/repair
What is significant of the element manganese? (2)
-blood clotting
-immune responses
What is significant of the element chromium?
improves insulin effect
What is significant of the element molybdenum? (2)
-breaking down toxic substances
-processing proteins
What types of bonds make up a single bond?
1 sigma bond
What types of bonds make up a double bond?
1 sigma bond AND 1 pi bond
What types of bonds make up a triple bond?
1 sigma bond AND 2 pi bonds
What is the electronegativity difference in a nonpolar covalent bond?
<0.5
What is the electronegativity difference in a polar covalent bond?
0.5-1.7
What is the electronegativity difference in an ionic bond?
>0.7
Is a bond between carbon and hydrogen polar or nonpolar?
polar
Is a bond between carbon and carbon polar or nonpolar?
polar
Is a bond that includes N, O, or P more likely to be polar or nonpolar?
nonpolar
In biochemistry, what three elements tend to form hybrid orbitals?
C, N, O
How do you determine hybridization?
-count the total number of bonds on the atom (double and triple bonds count as ONLY 1, do not consider the type of bond)
-lone pairs count as 1
What is the hybridization state of an atom with 1 group?
s
What is the hybridization state of an atom with 2 groups?
sp
What is the hybridization state of an atom with 3 groups?
sp²
What is the hybridization state of an atom with 4 groups?
sp³
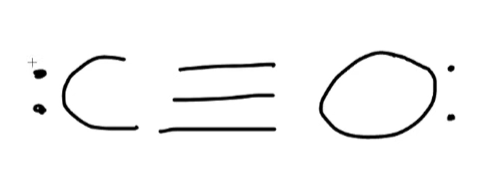
What is the hybridization state of the carbon atom in this molecule?
sp
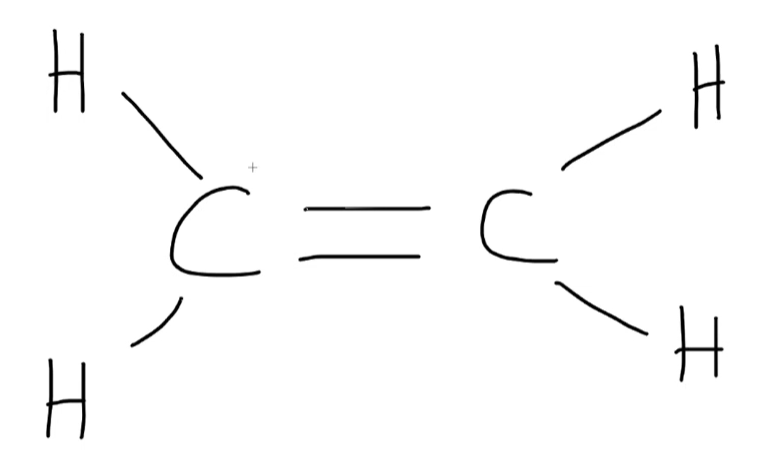
What is the hybridization state of the carbon atom in this molecule?
sp²