Ch 6: Chemical Bonding Theories
1/59
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
60 Terms
Valence Bond Theory
chemical bond - results from overlap of two half-filled orbitals and the spin-pairing of electrons
also from overlap of empty orbital with orbital containing lone pair
geometry of overlapping orbitals determines molecular shape
valence e- in standard s, p, or d atomic orbitals or in hybrid orbitals
forming bonds from atomic orbitals alone…
…does not procude the expected compounds (theoretical prediction)
Atomic orbitals on carbon…
…mix or hybridize so that mor C-H bond can form
lowers energy of the molecule
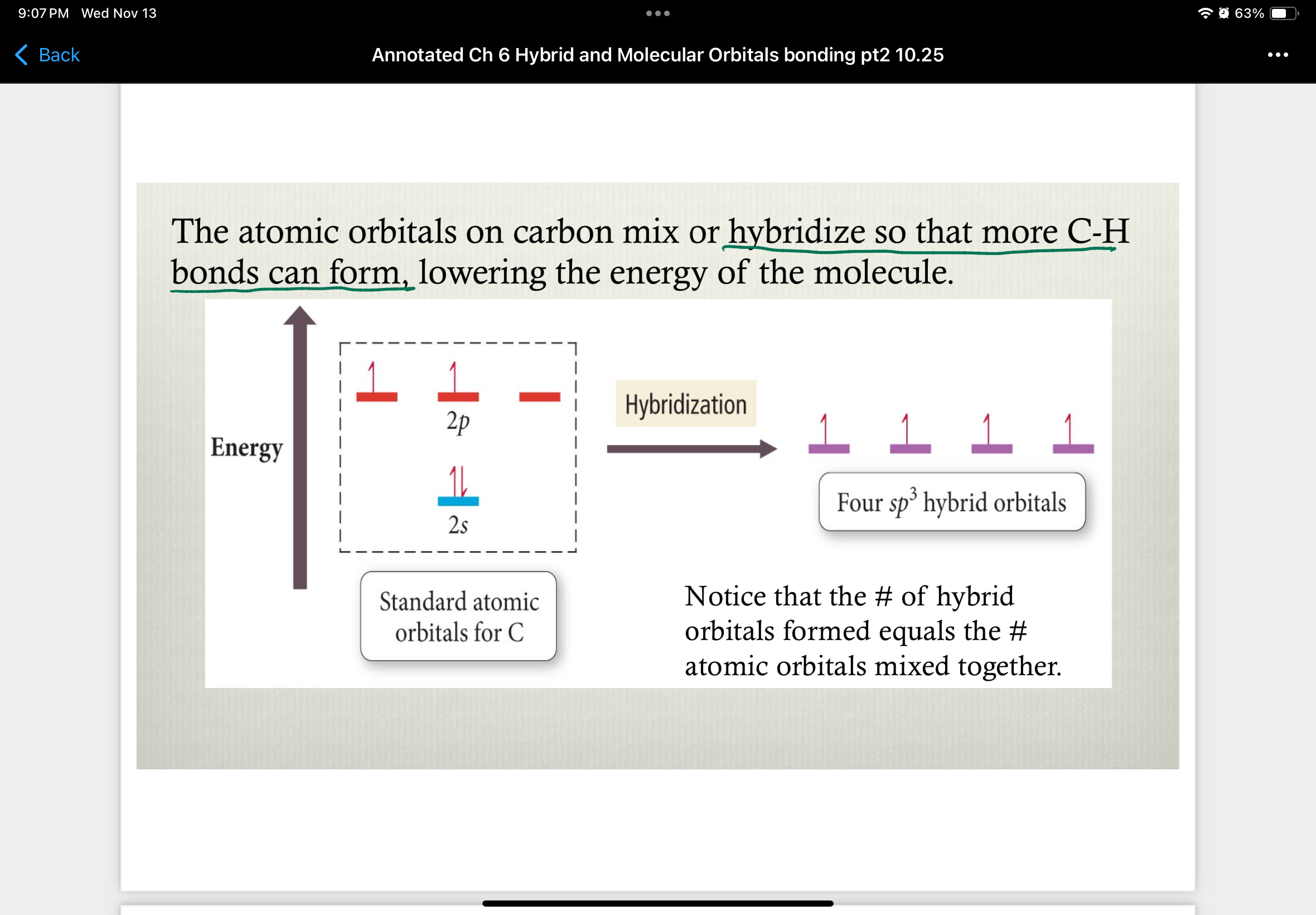
number of hyrbid orbitals formed =
number of atomic orbitals mixed together
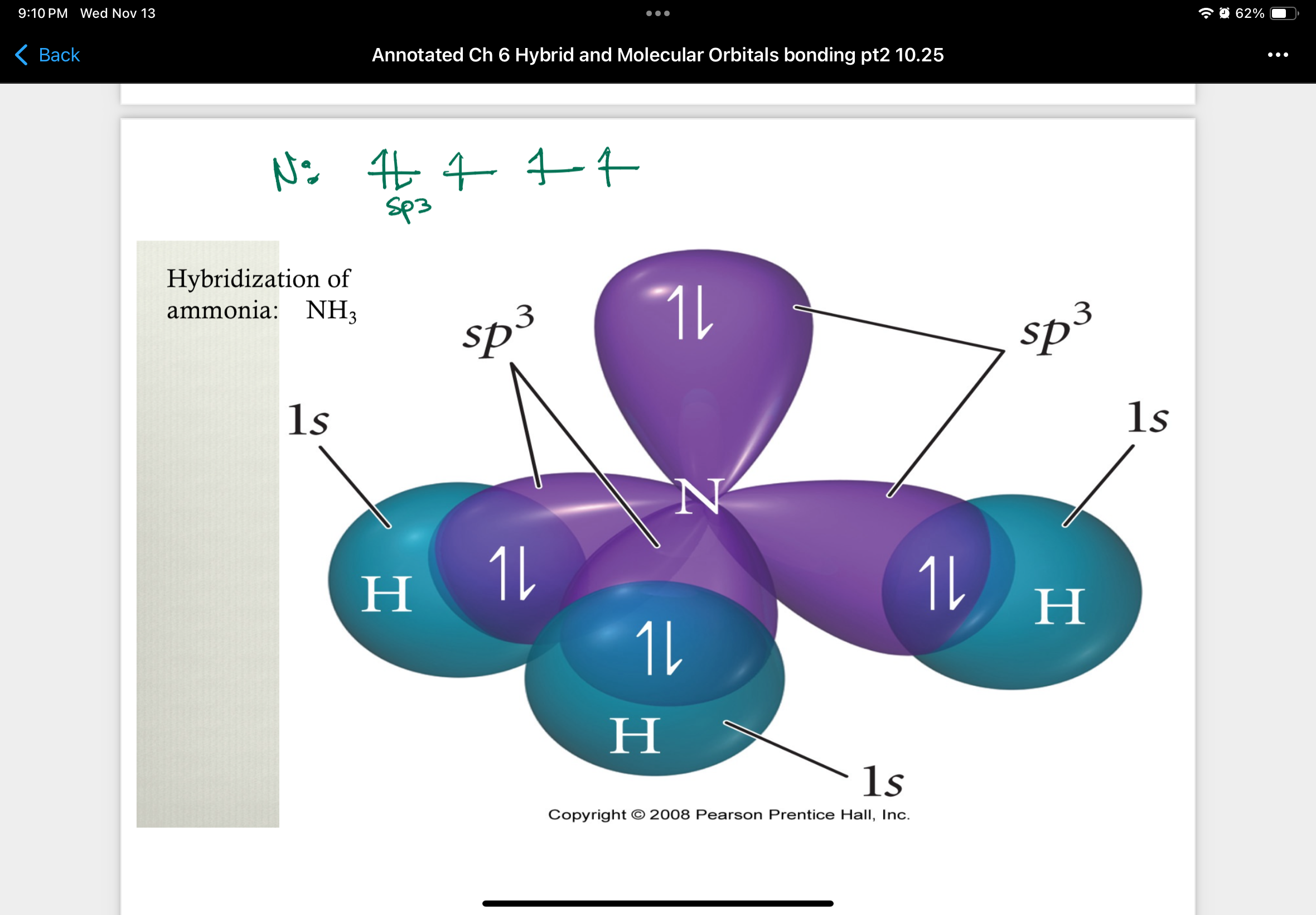
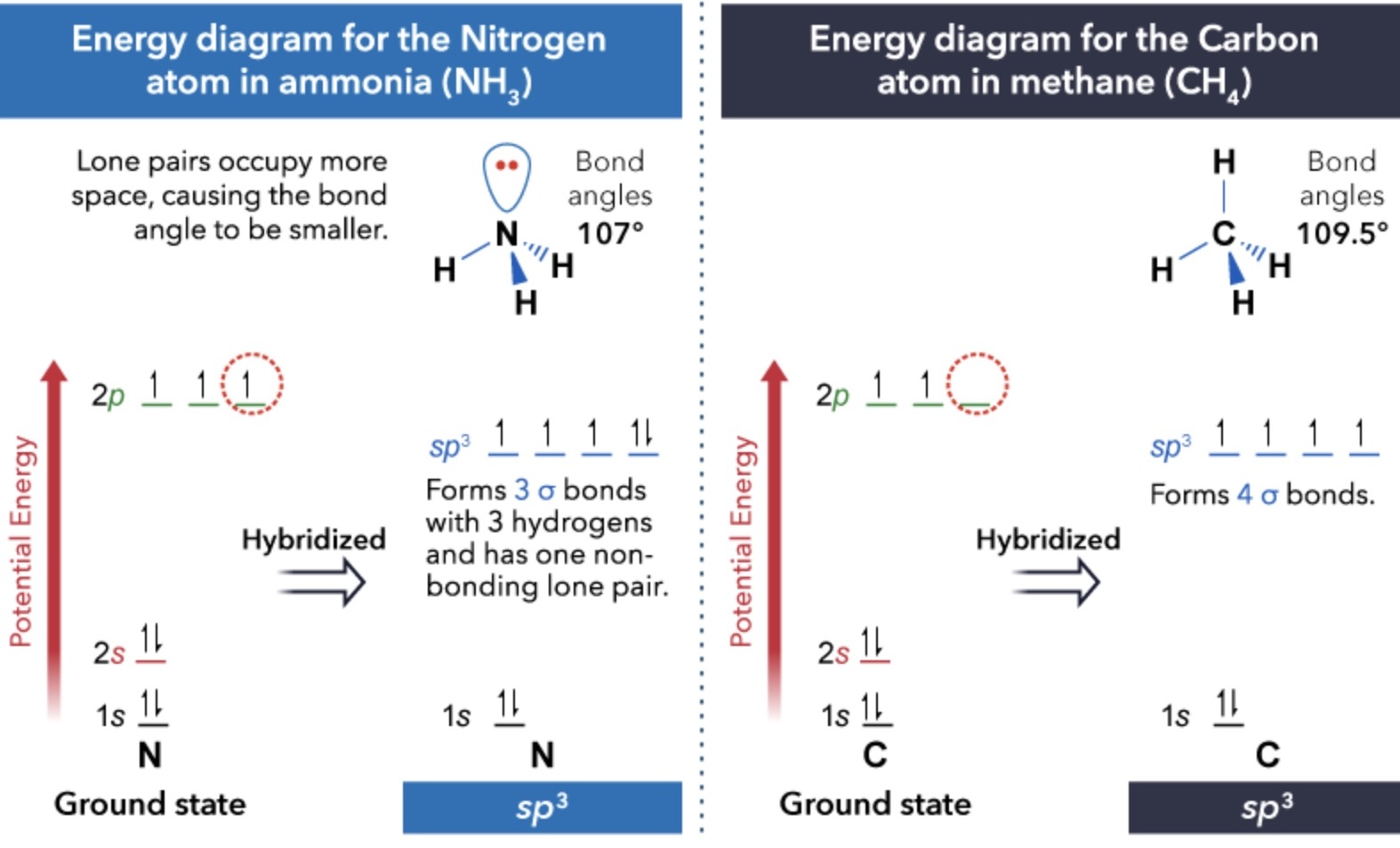
Hybridization of ammonia: NH3
If px and py orbitals are mized with s-orbitals to form hybrid orbitals…
…then the sp2 orbitals are all in the xy plane
this leaves a pz atomic orbital perpendicular to the sp2 orbitals
pz orbital left unchanged
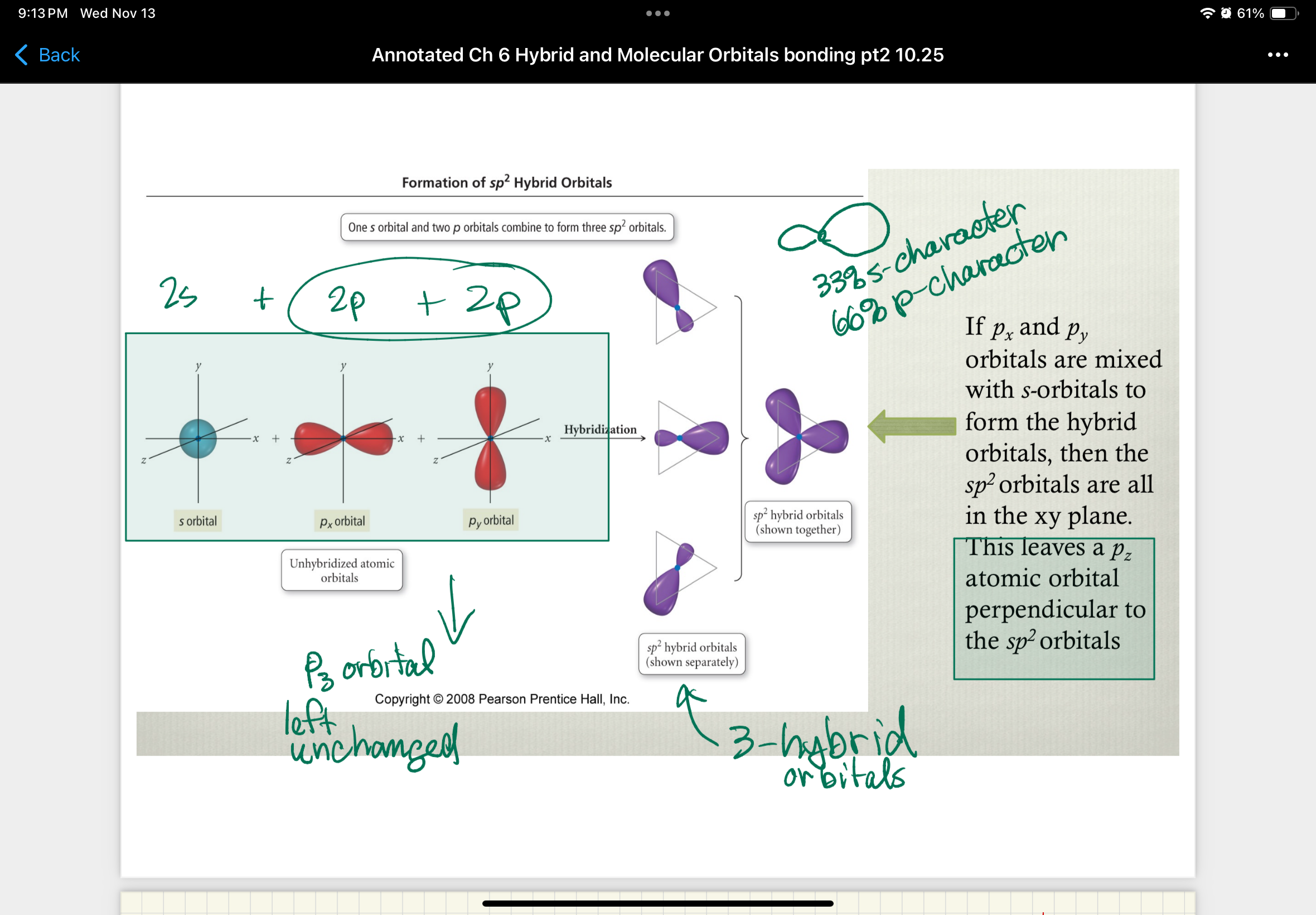
In forming sp2-hybrid orbitals - the s-orbital is mixed with only…
…2 of the 3 p-orbitals
after hybridization there are three sp2-hybrid orbitals and one p-orbital left unchanged
unhybridized p-orbitals can be used to…
form either pi bonds or sigma bonds
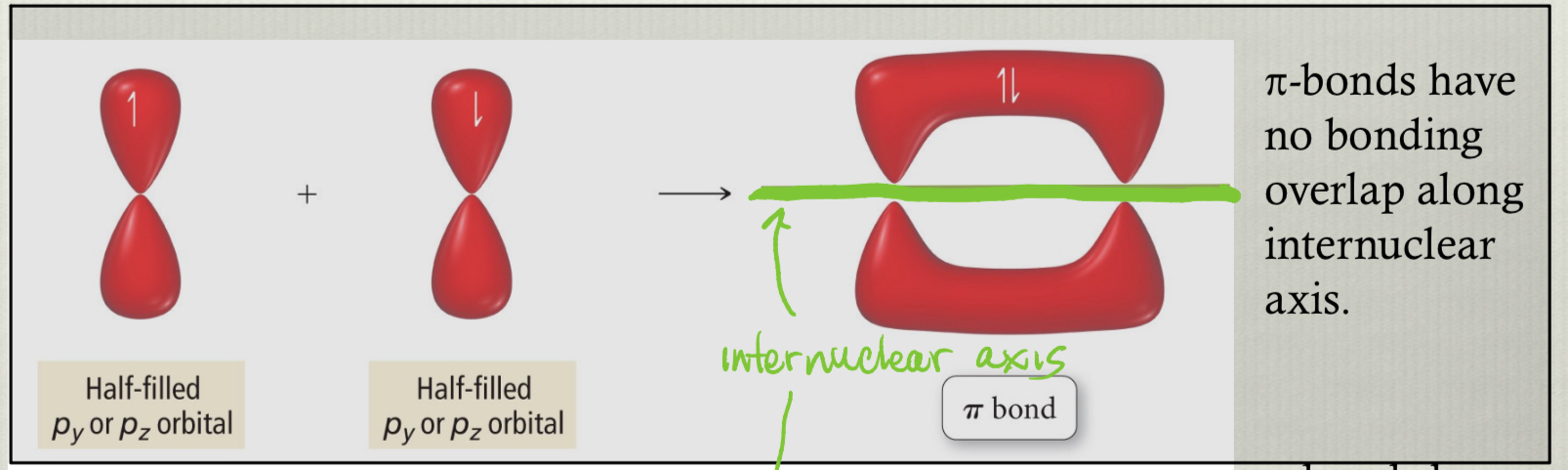
pi bonds
have no bonding overlap along internuclear axis
usually formed from unhybridized p-orbitals
distinct from σ bonds because they possess a lobe above and below the internuclear region
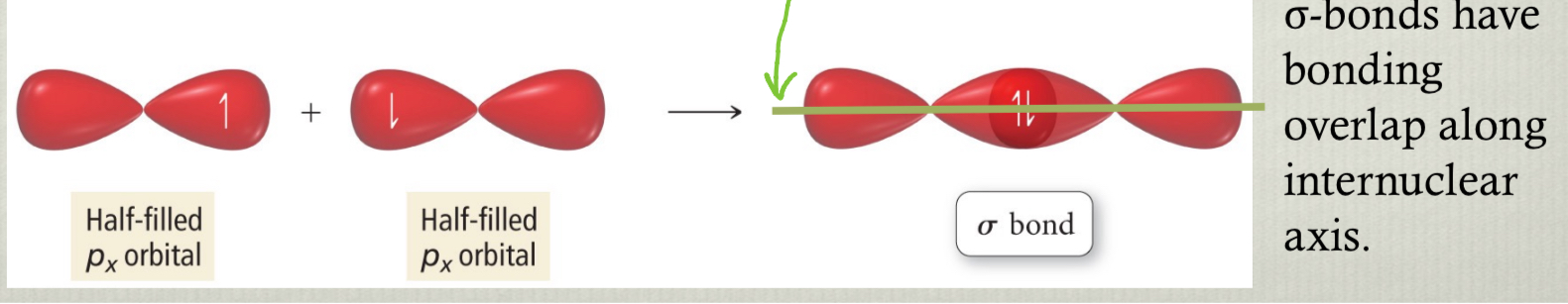
sigma bonds
have bonding overlap along internuclear axis
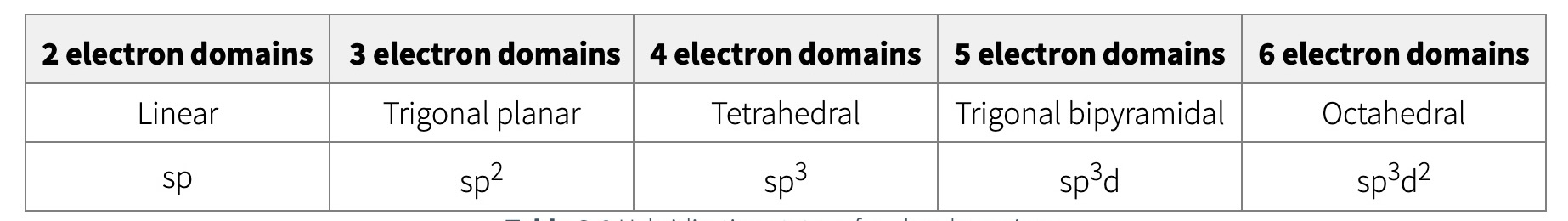
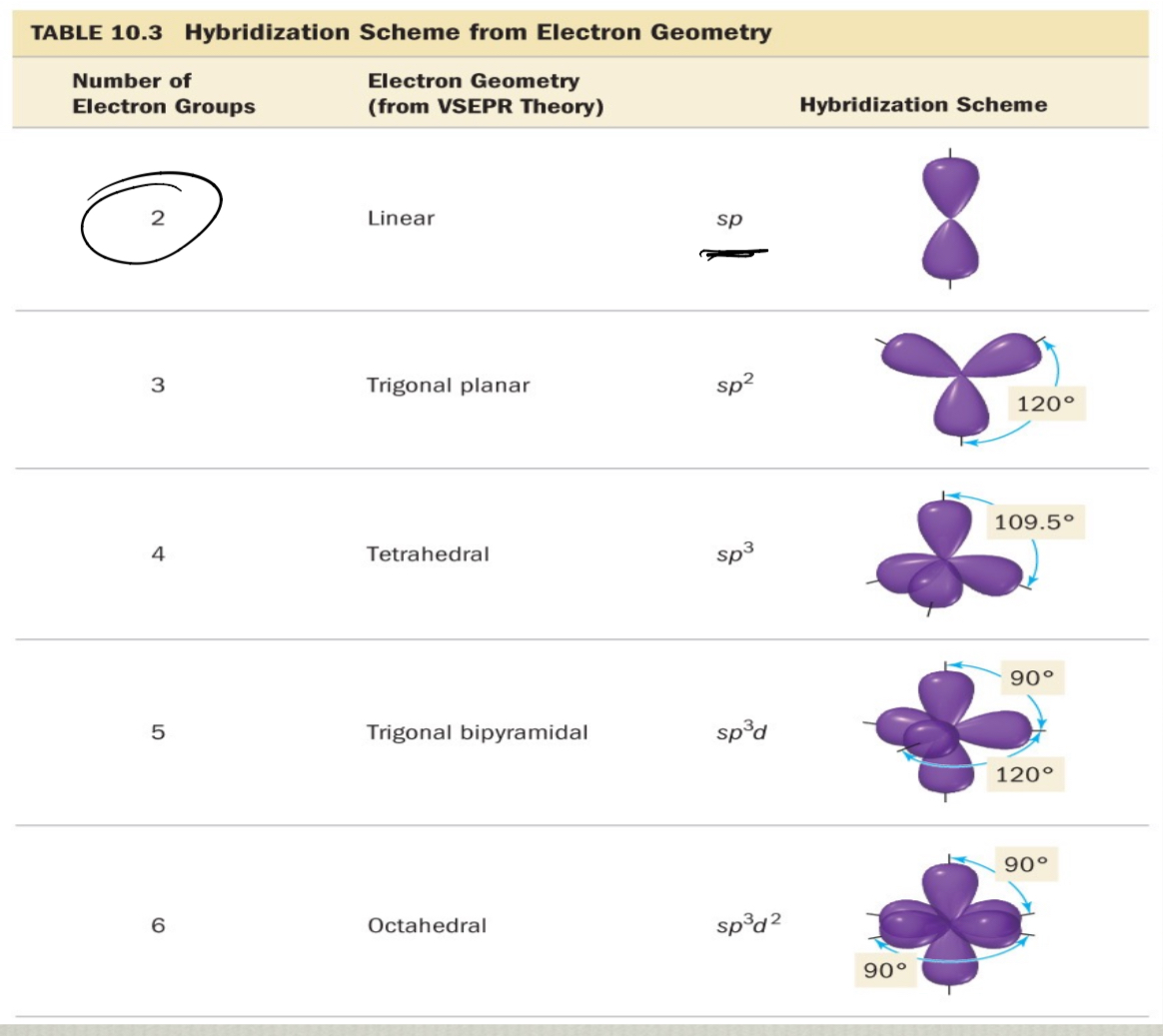
Determining hybridization state
depends on electron geometry
electron geometry does not determine the actual shape though
Electron lone pairs can…
…also reside in orbitals
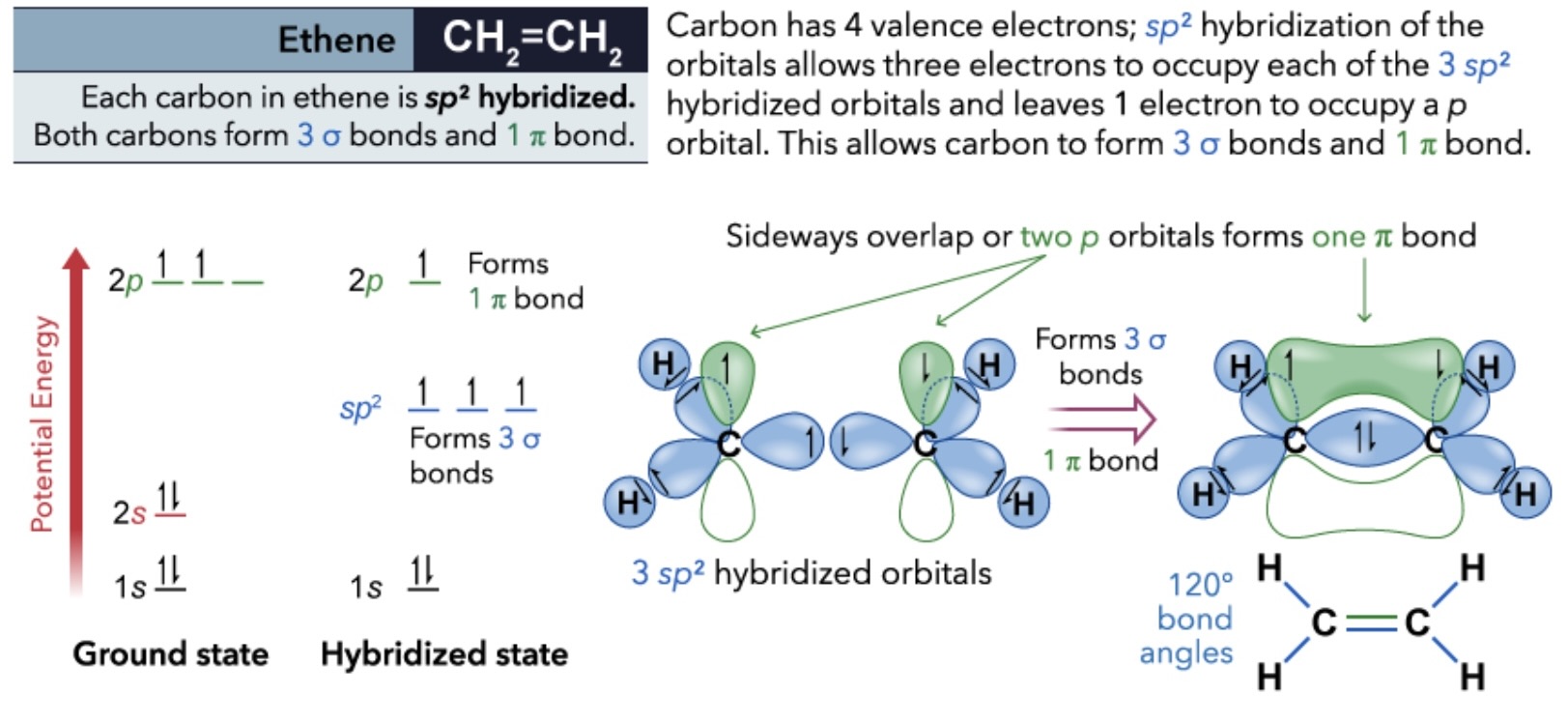
Ethene sp2 hybridization
The C-C bond formed as a result of the C(sp2)-C(sp2) overlap is called a σ bond
results from head-on overlap and has cylindrical symmetry
The second C-C bond is called a π bond and results from the side-on overlap of the remaining, unhybridized 2p orbital in each C atom
rotation of pi bonds
location of e- would move and the bond could break if roataion is severe enough
energy (light or temp) is required to break the bond before rotation can occur
restricted rotation of pi bonds
rotation does not occur at normal temps
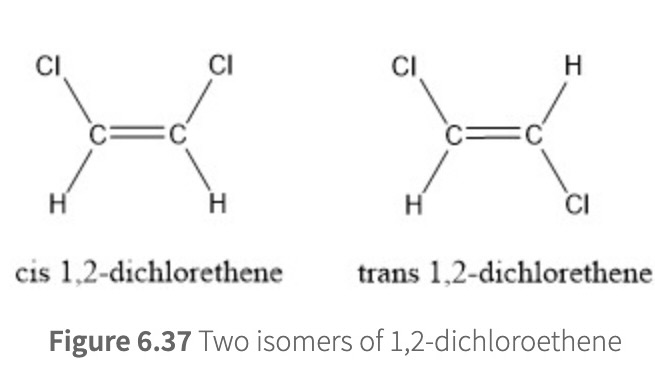
one important consequence of restricted rotation is that different isomers (w/ diff molecular properties) can exist for molecules with pi bonds
ex: 1,2-dichloroethene
two forms locked in given configurations
trans isomer = non-polar
cis isomer = polar
strength of sigma bonds vs. pi bonds
sigma bonds are STRONGER than pi bonds
pi bonds is weaker because side-on overlap leads to smaller increase in shared electron density
electron density in a pi bond is more spread out, making it less effective at holding the nuclei together
head-on overlapping of sigma bonds = less internuclear respulsion bc of higher concentration of e- between nuclei
side-on overlapping of pi bonds = more internuclear repulsion bc of smaller concentration of e- (more spread out) between nuclei
pi = less stable and weaker
double bond consists of…
…one sigma bond and one pi bond
free rotation of sigma bonds
because of the spherical symmetry around the internuclear axis - can rotate freely without altering chemical bond
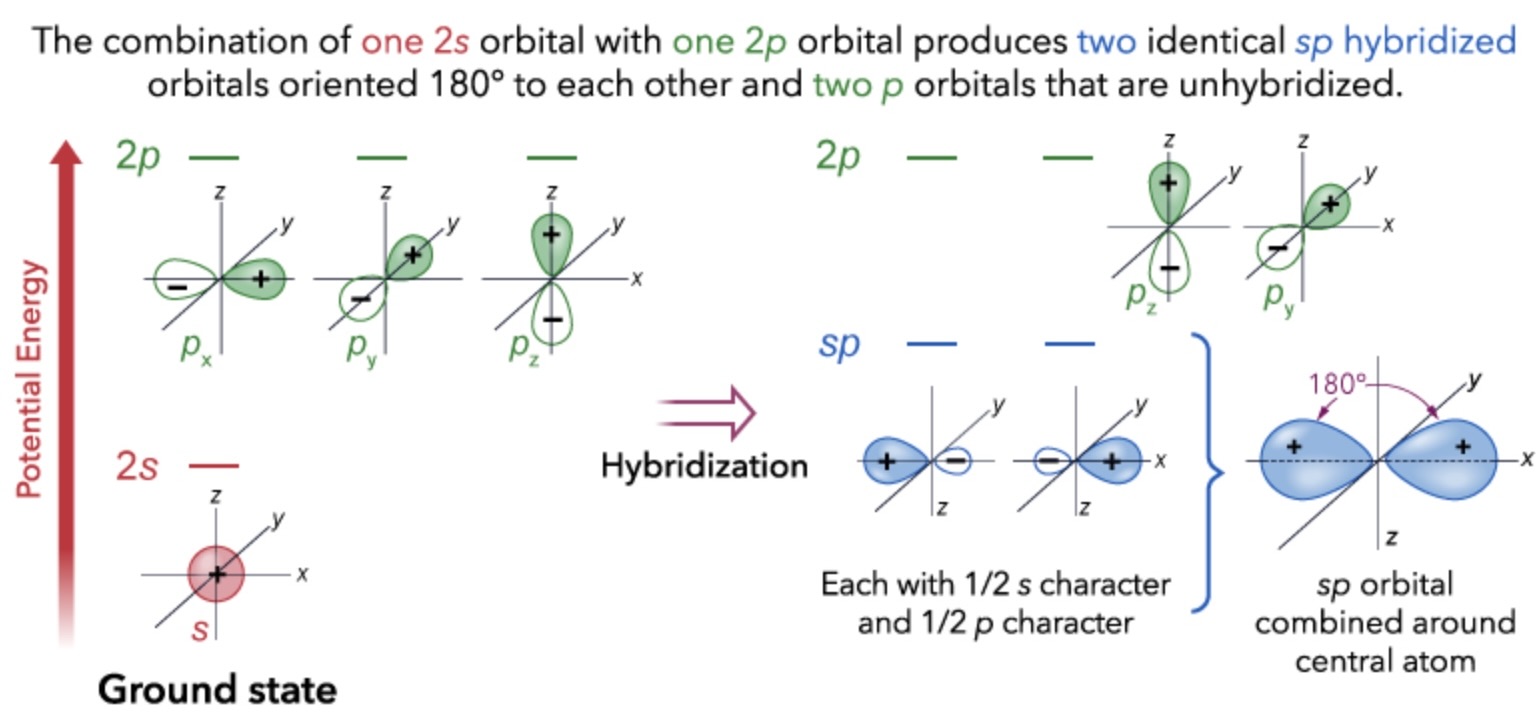
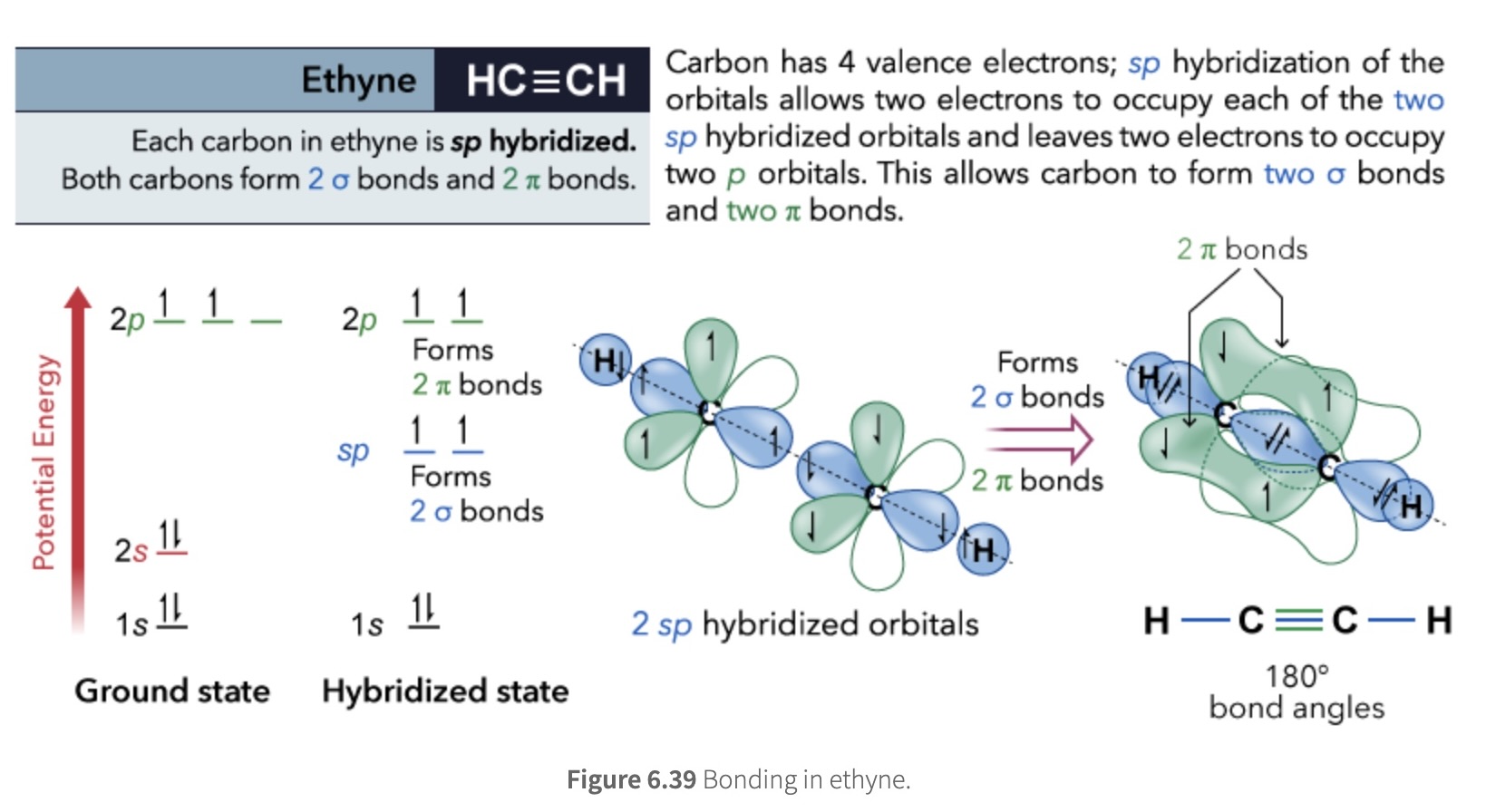
sp hybridization
one s and one p orbital combine to form two sp orbitals
2 unhybridized p orbitals left (pi bonds)
used to describe the bonding and linear molecular geometry of alkynes
alkynes have a triple C-C bonds
ex: Ethyne
C-H σ bonds are formed by C(sp)-H(1s) overlap
C-C σ bond is formed by C(sp)-C(sp) overlap
C-C π bonds are formed from side-on overlap of the unhybridized 2p orbitals on each carbon atom and are orthogonal to each other
Ethyne (sp hybridization)
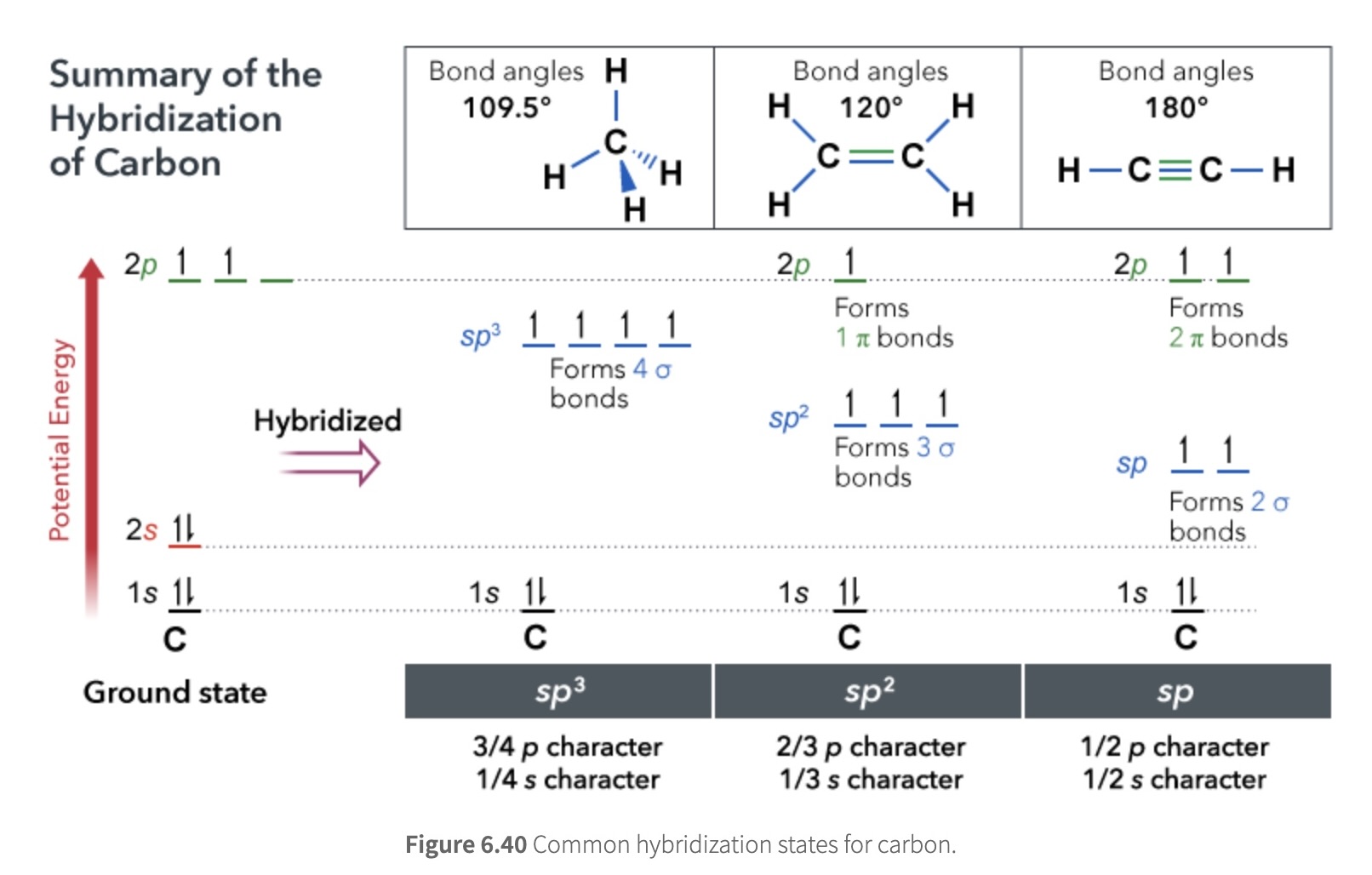
Summary of the Hybridization of Carbon
σ bonds always involve the head-on overlap of hybrid orbitals (except with hydrogen, which uses its 1s orbital to make bonds).
π bonds always result from side-on overlap of unhybridized orbitals.
Lone pairs always reside in hybrid orbitals.
triple bonds
composed of one sigma bond and two pi bonds
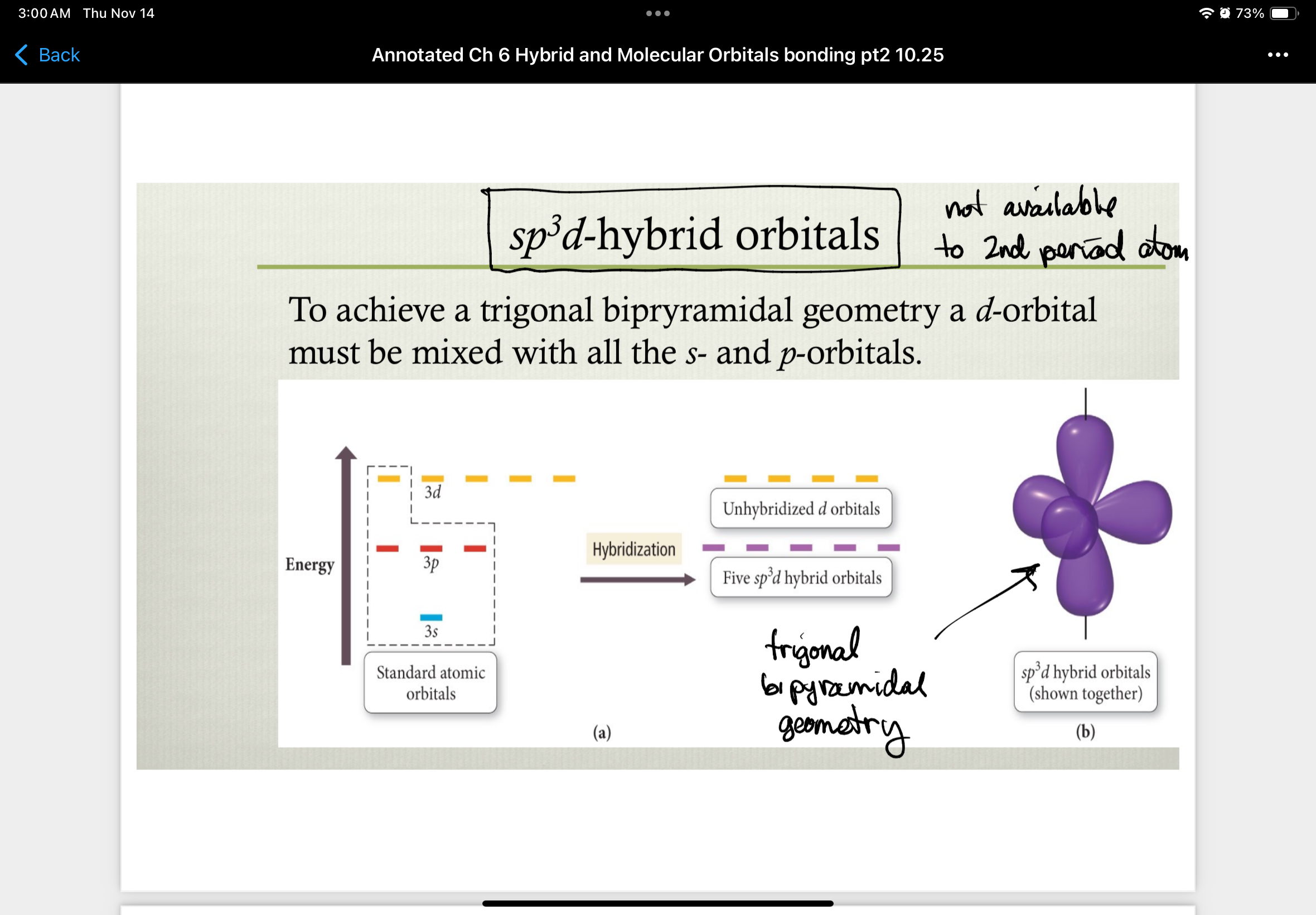
sp3d-hybrid orbitals
to achieve trigonal bipyramidal geometry a d-orbital must be mixed with all the s- and p- orbitals
not available to 2nd period atom
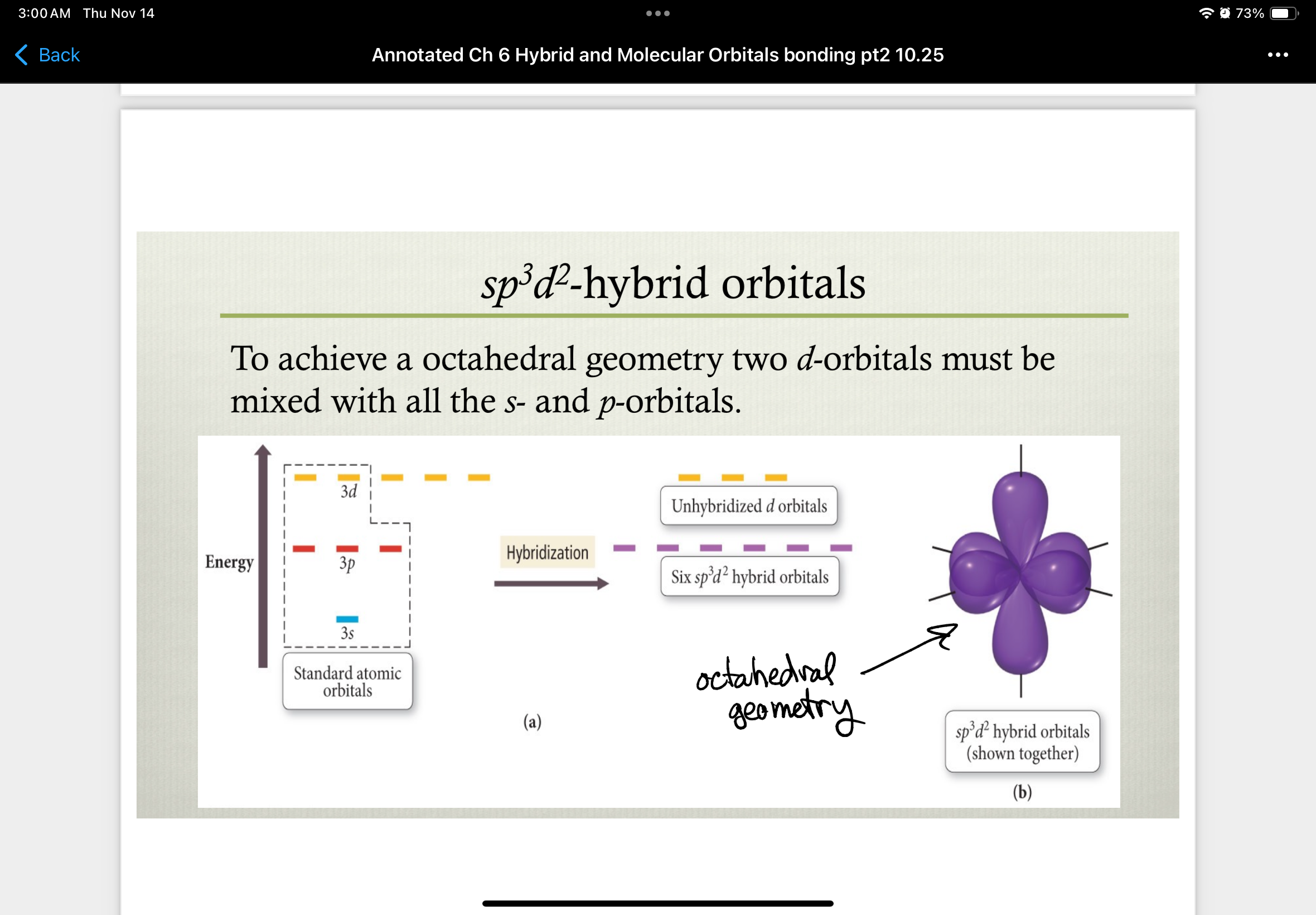
sp3d2-hybrid orbitals
to achieve an octahedral geometry two d-orbitals must be mixed with all the s- and p-orbitals
to determine the hybridization of an atom:
draw the correct Lewis structure
determine the steric # (or # of electron groups)
the geometry of the steric # determines the hybridization
cannot use the same p-orbital…
for 2 diff pi bonds
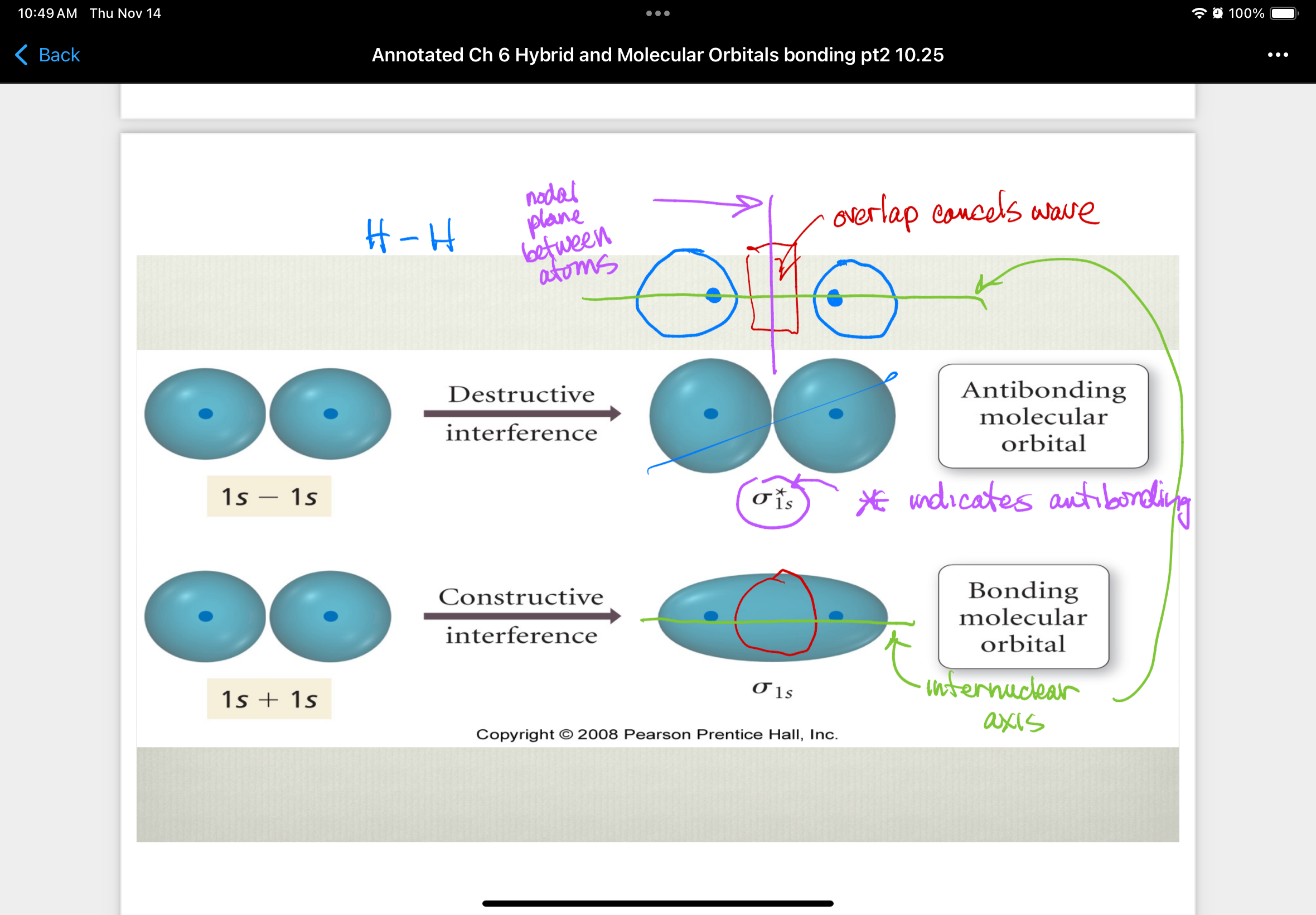
Antibonding molecular orbital
orbitals that are produced from destructive interference of atomic wave functions that remove electron density between two atomic nuclei
higher in energy than parent atomic orbitals = destabilize molecule
e- in antibonding orbitals are excluded from internuclear region
e- in antibonding orbitals have a lower probability of being near the nuclei than they did when they were part of an individual atom
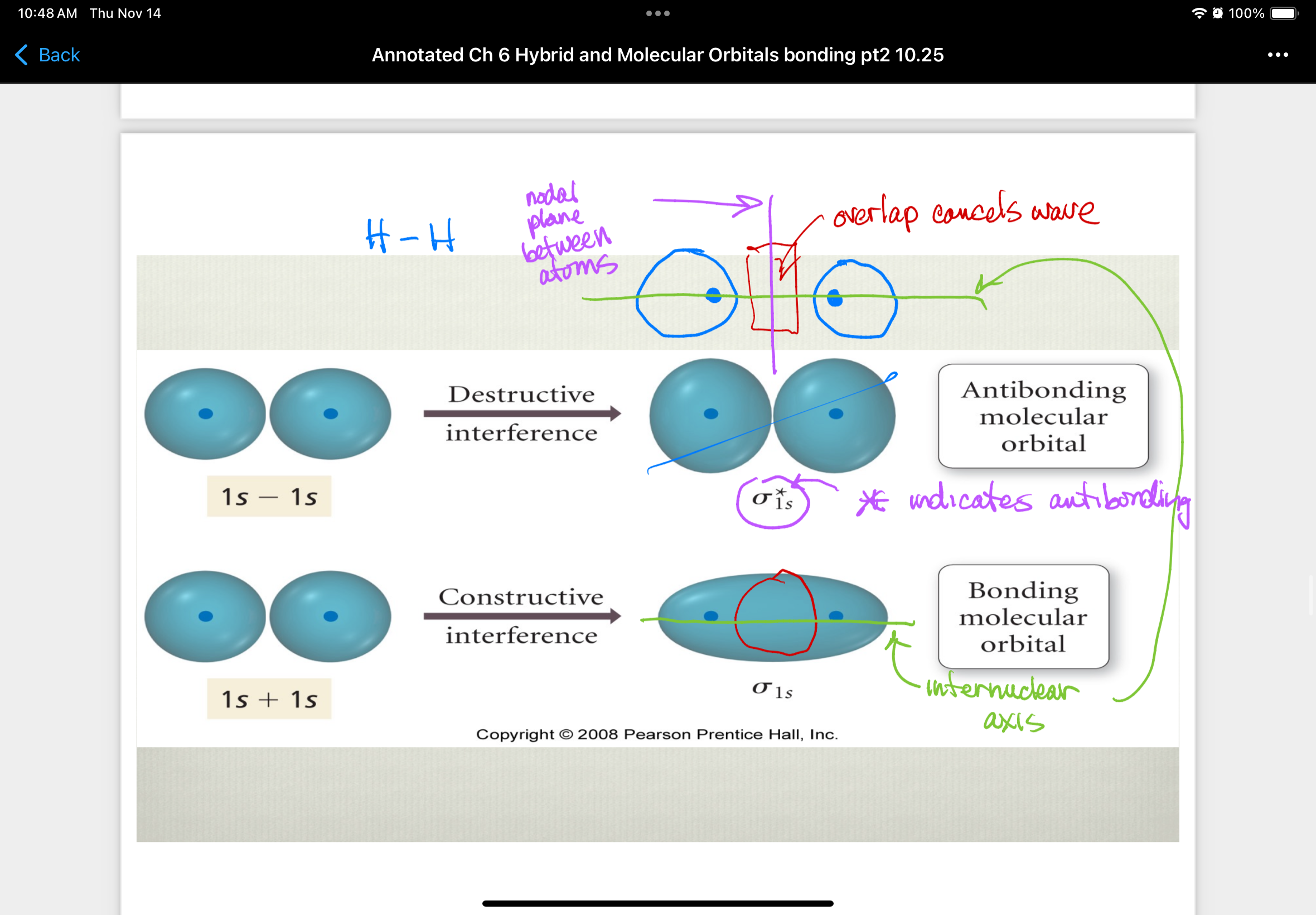
Bonding molecular orbital
orbitals that are produced from constructive interference of atomic wave functions that place electron density between the two nuclei
overlap regions must align as they approach each other on axis
lower in energy than parent atomic orbitals = e- in these orbitals makes molecule more stable than individual atoms
e- in bonding orbitals spend time in internuclear region and are attracted to both nuclei
MO diagram of HF
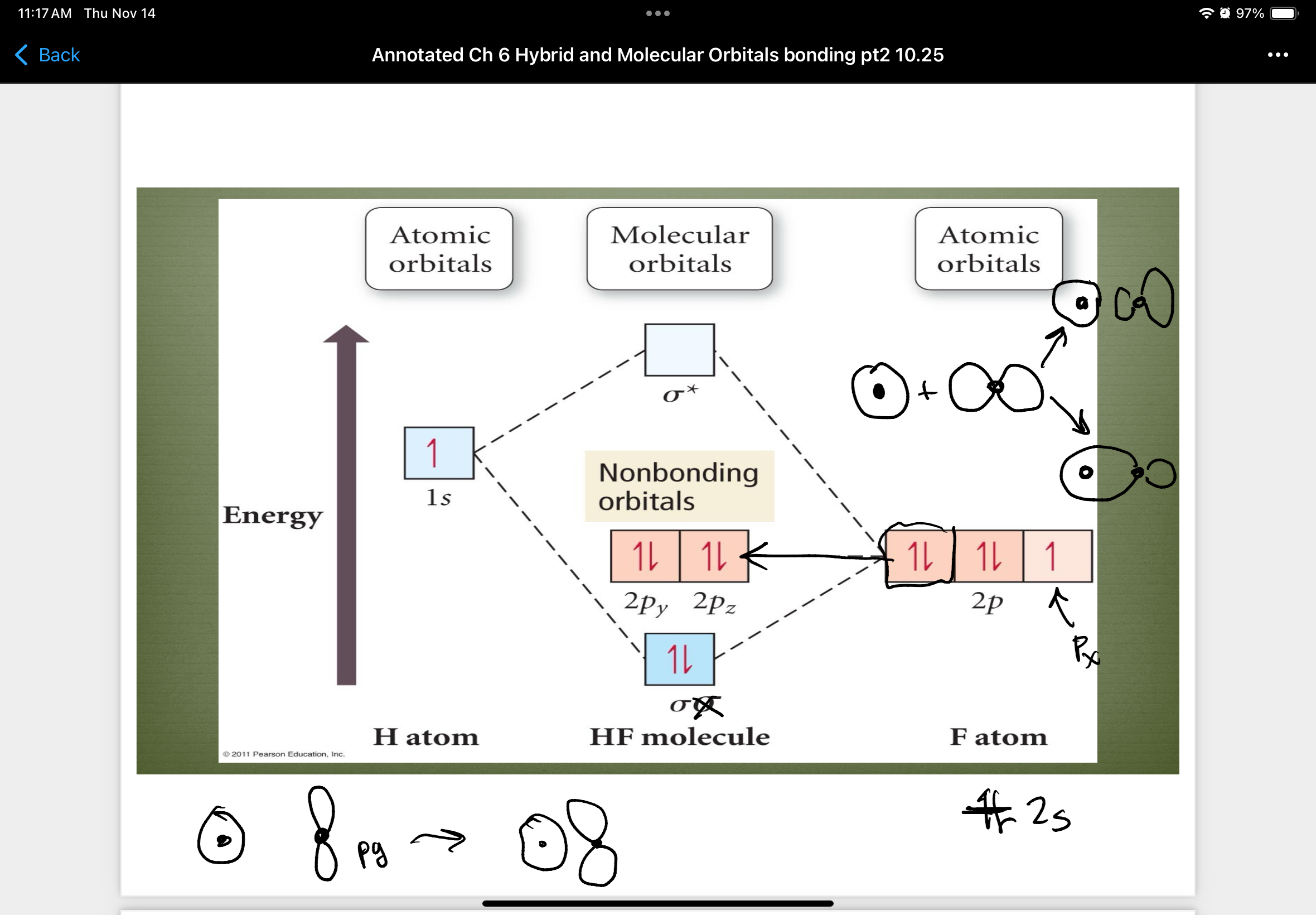
Once you form the molecular obital…
…then the atomic orbitals no longer exist
Each MO can hold:
2 e-
MO orbitals filled using Aufbau Principle
Orbitals are conserved
Atomic orbitals in = molecular orbitals out
Bond Order (BO)
BO = ((# of e- in bonding orbitals) - (# of e- in antibonding orbitals))/2
greatest stabilization in bonding MO:
occurs when the original orbitals have the correct symmetry and the original orbitals have similar energies
combinations of 2px atomic orbitals
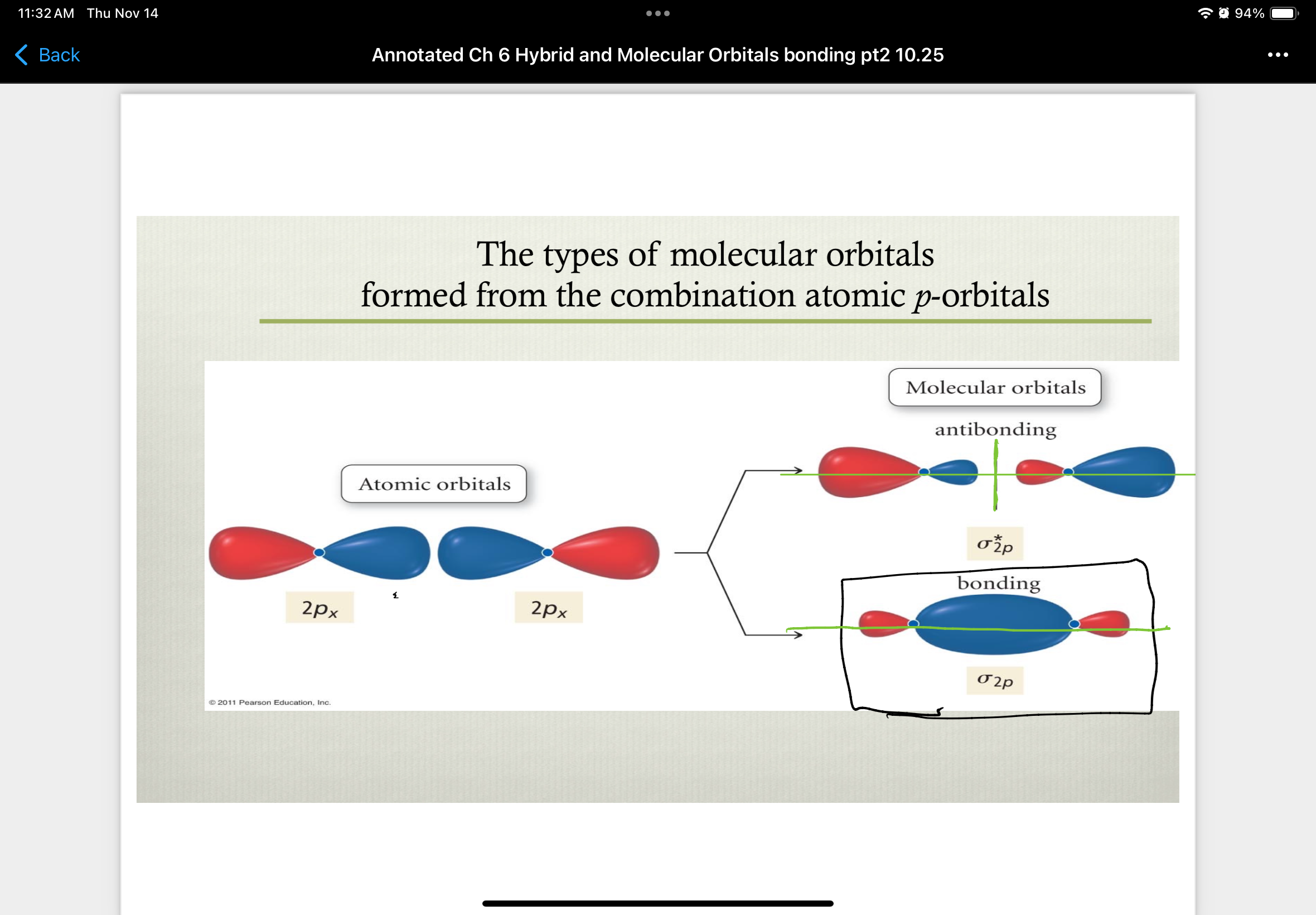
combinations of 2py atomic orbitals
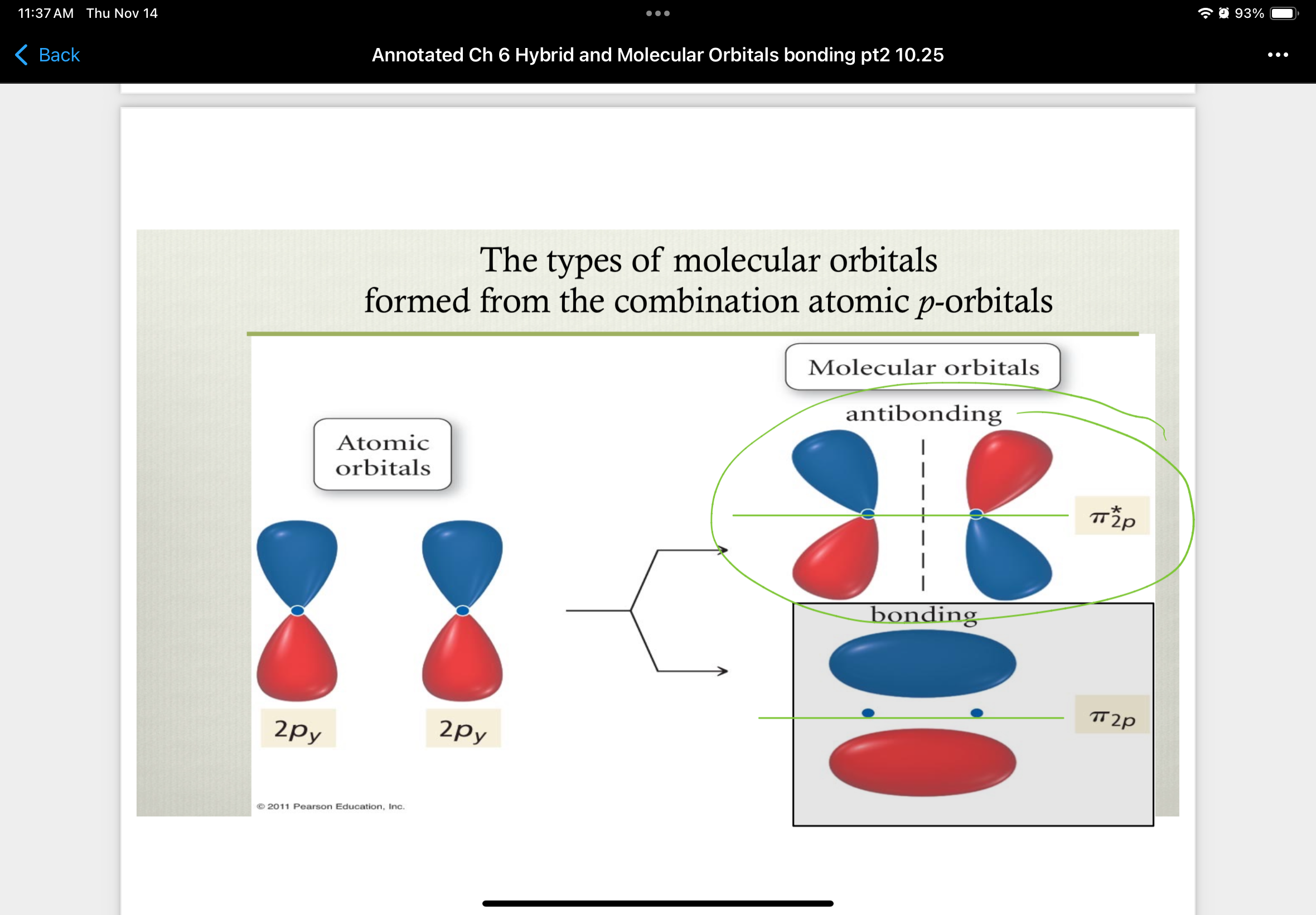
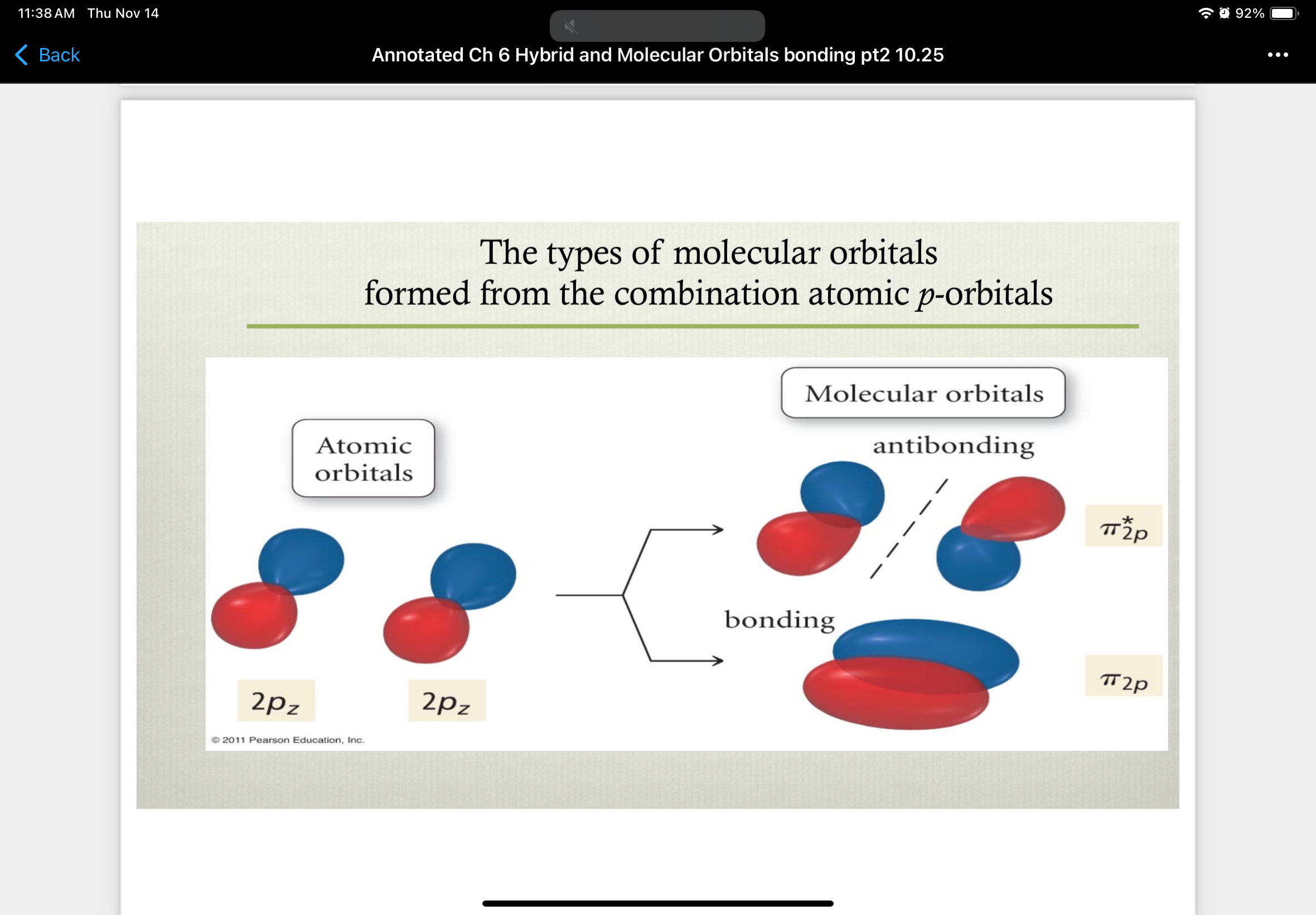
combinations of 2pz atomic orbitals
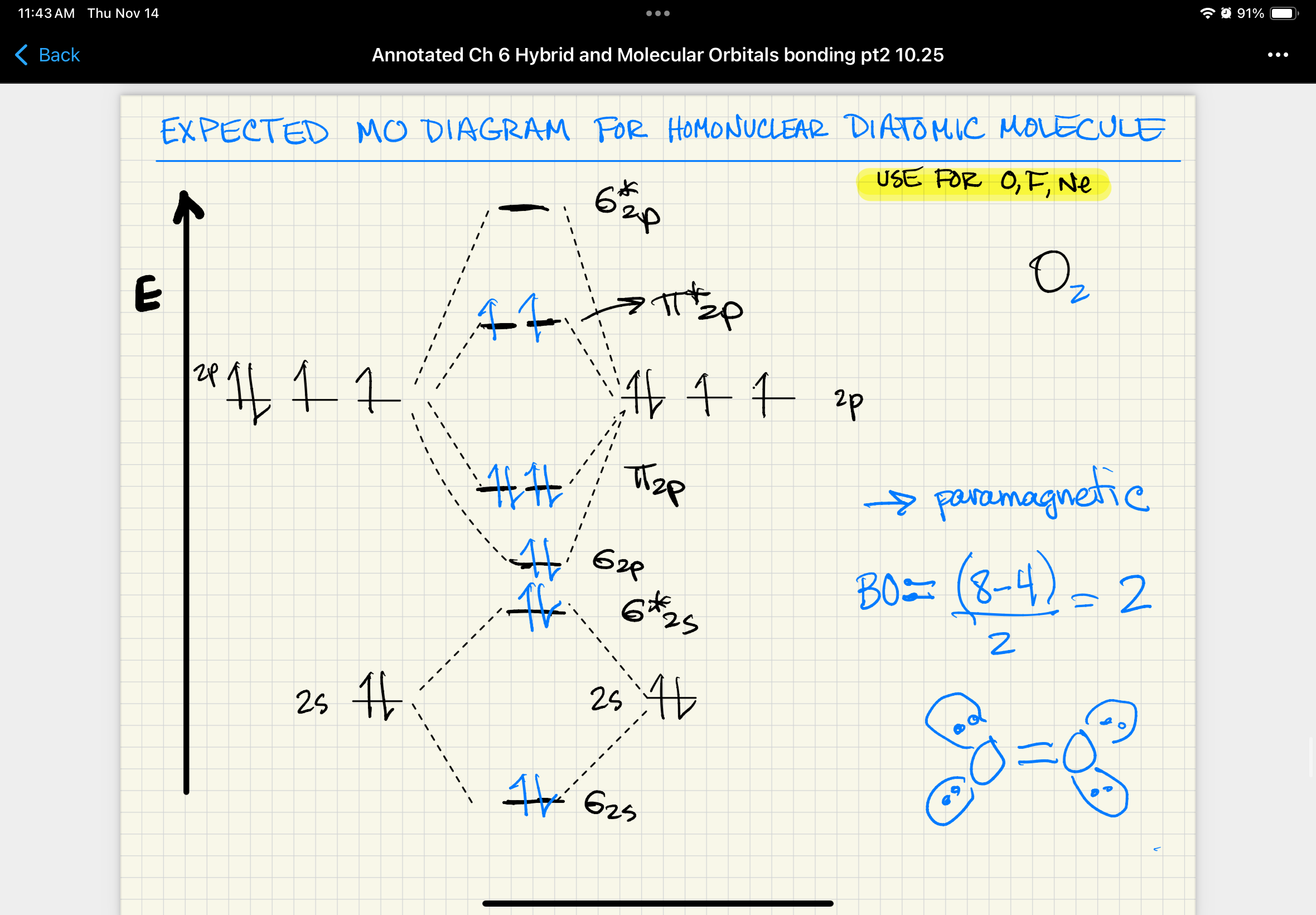
expected MO diagram for homonuclear diatomic molecule (O, F, Ne)
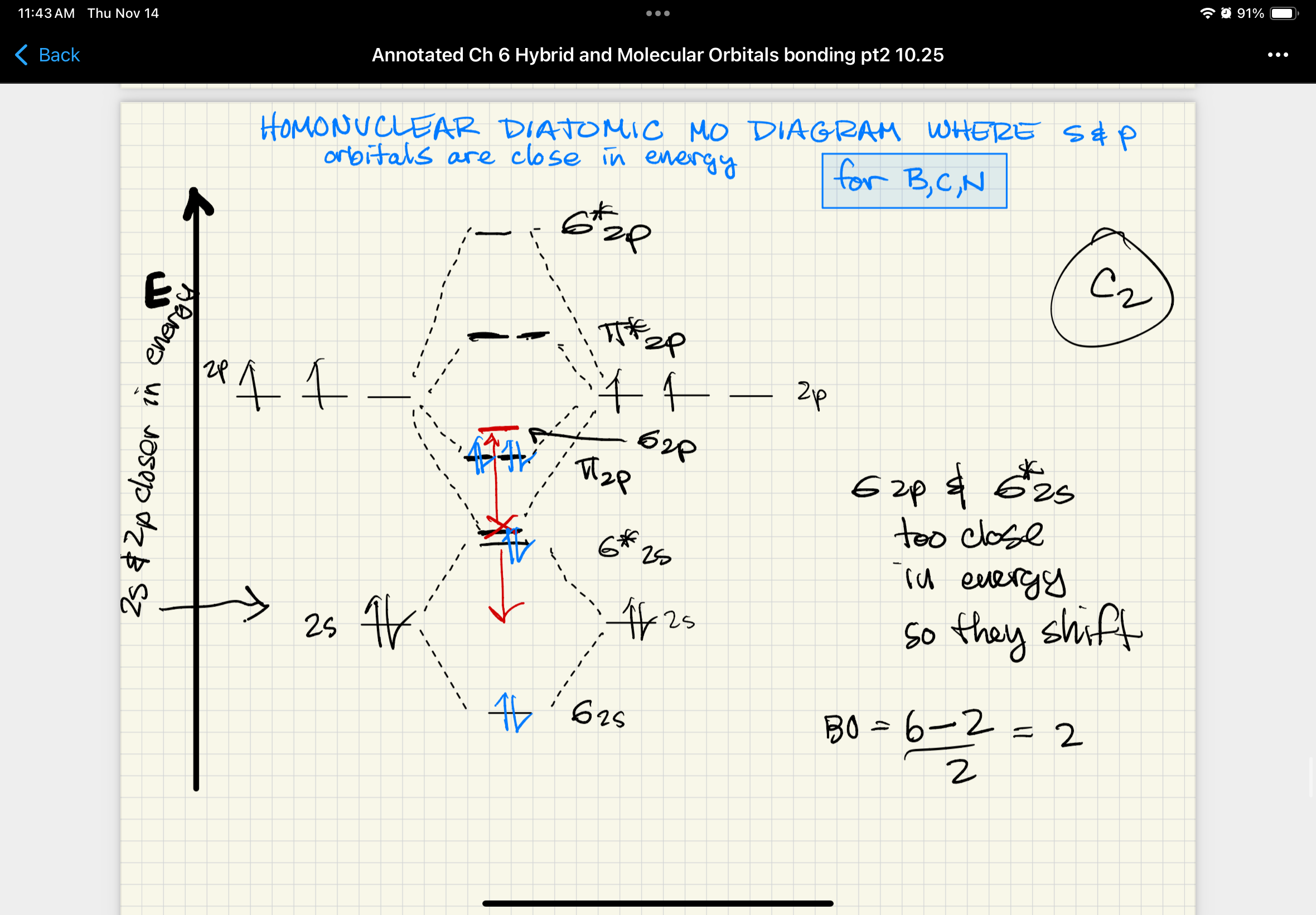
expected MO diagram for homonuclear diatomic molecule where s and p orbitals are close in energy (B, C, N)
Larger bond order =
shorter bond = stronger bond
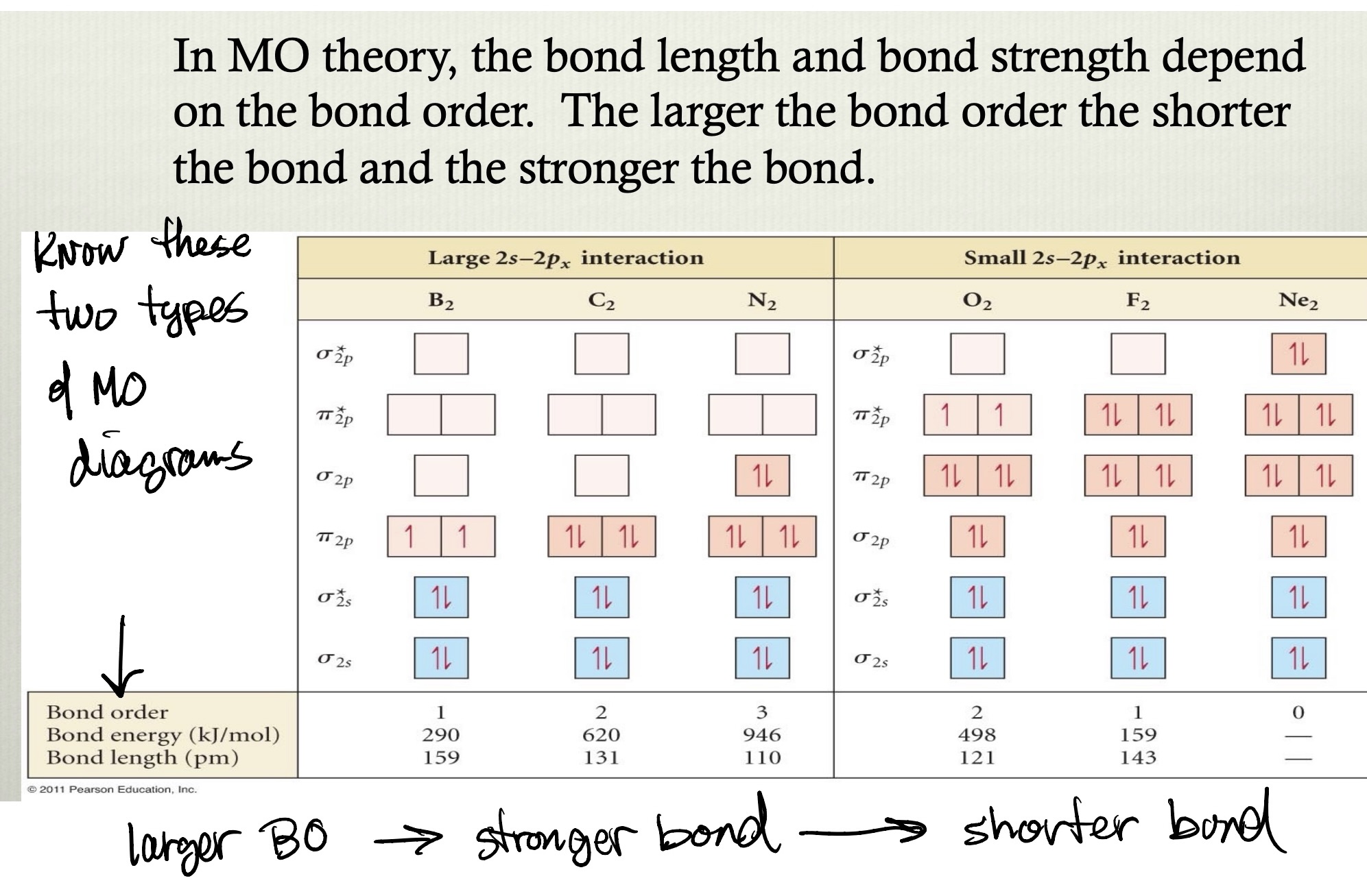
MO diagram for NO
energies of O-atomic orbitals are lower than that of the N-atomic orbitals
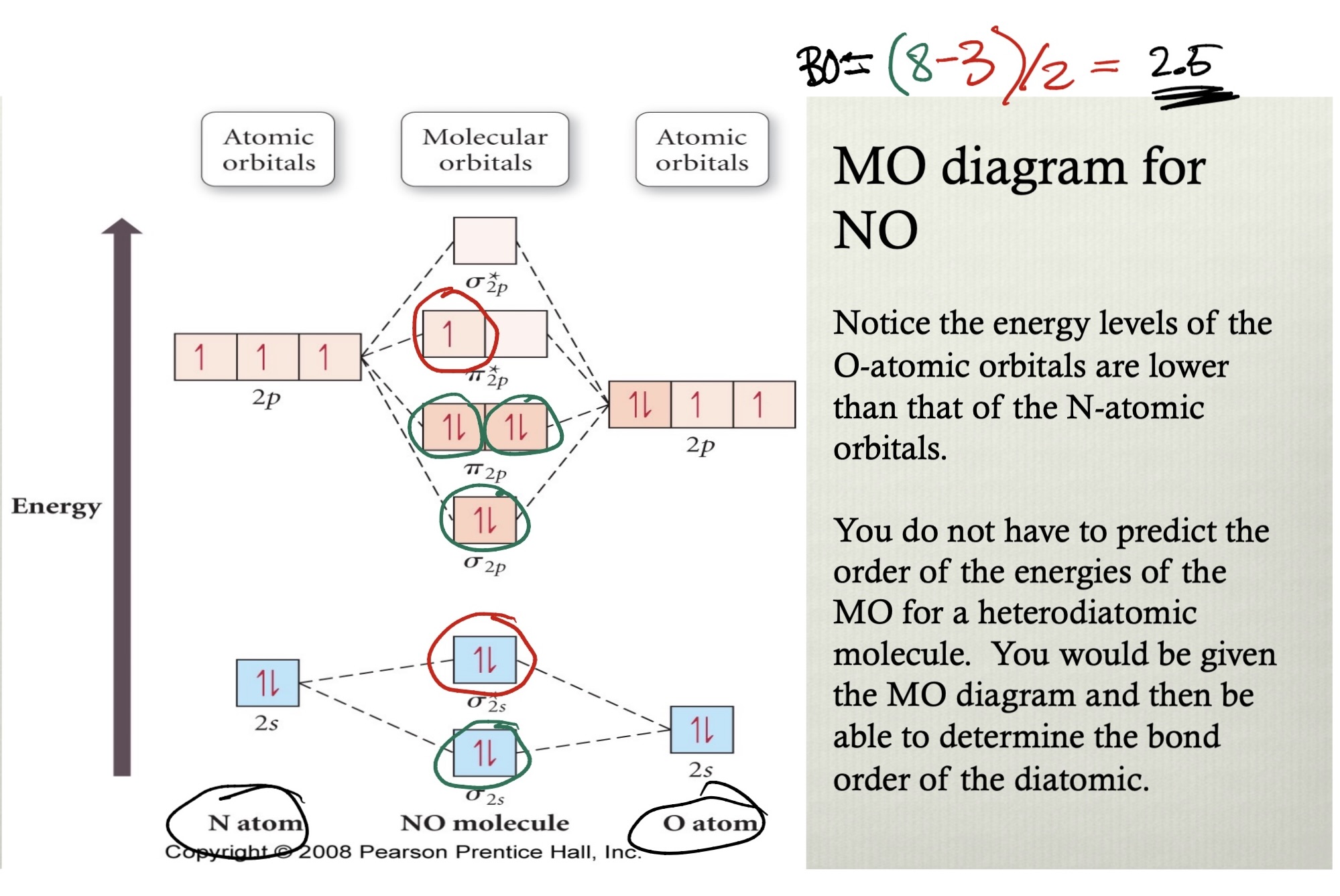
pi bonds are free to extend…
…over multiple atoms in molecular orbital theory
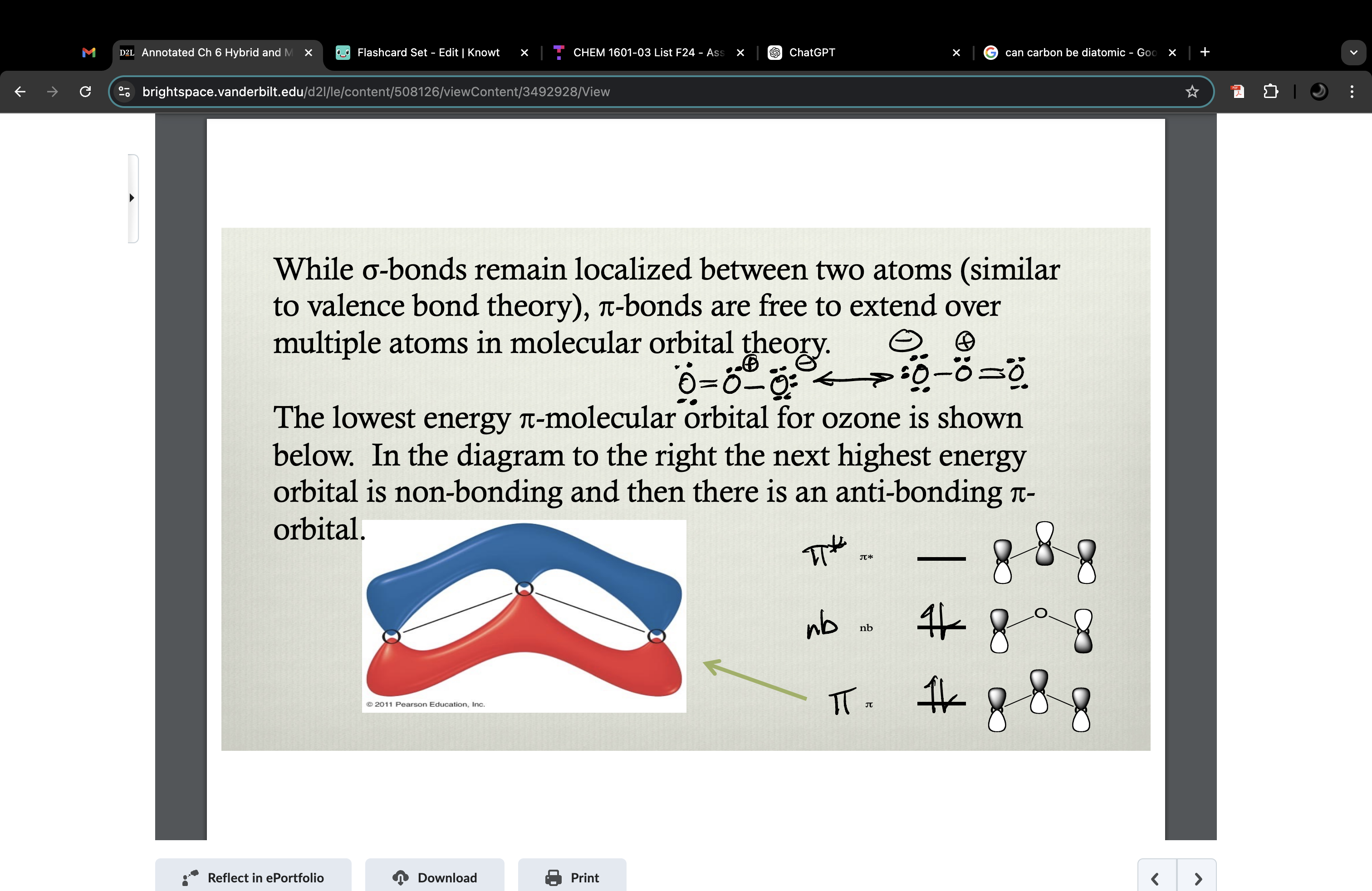
For molecules that do not have resonance…
…valence bond theory does a good job of describing the bonding because the bonding is localized between two atoms at a time
on the other hand, for a resonance structure of something like benzene, the valence bond theory does not adequately explain the bonding
highest-occupied molecular orbital (HOMO)
the highest energy molecular orbital which is fully filled
lowest-unoccupied molecular orbital (LUMO)
lowest energy molecular orbital which is empty
frontier molecular orbitals
HOMO and LUMO
these orbitals tend to be involved in bond making and breaking and, therefore, play major roles in predicting and explaining the outcomes of chemical reactions
sea of electrons model for metallic bonding
valence e- are delocalized and can travel freely over the entire sample of metallic solid
the valence e- do not “belong” to any particular metal ion but to the crystal as a whole
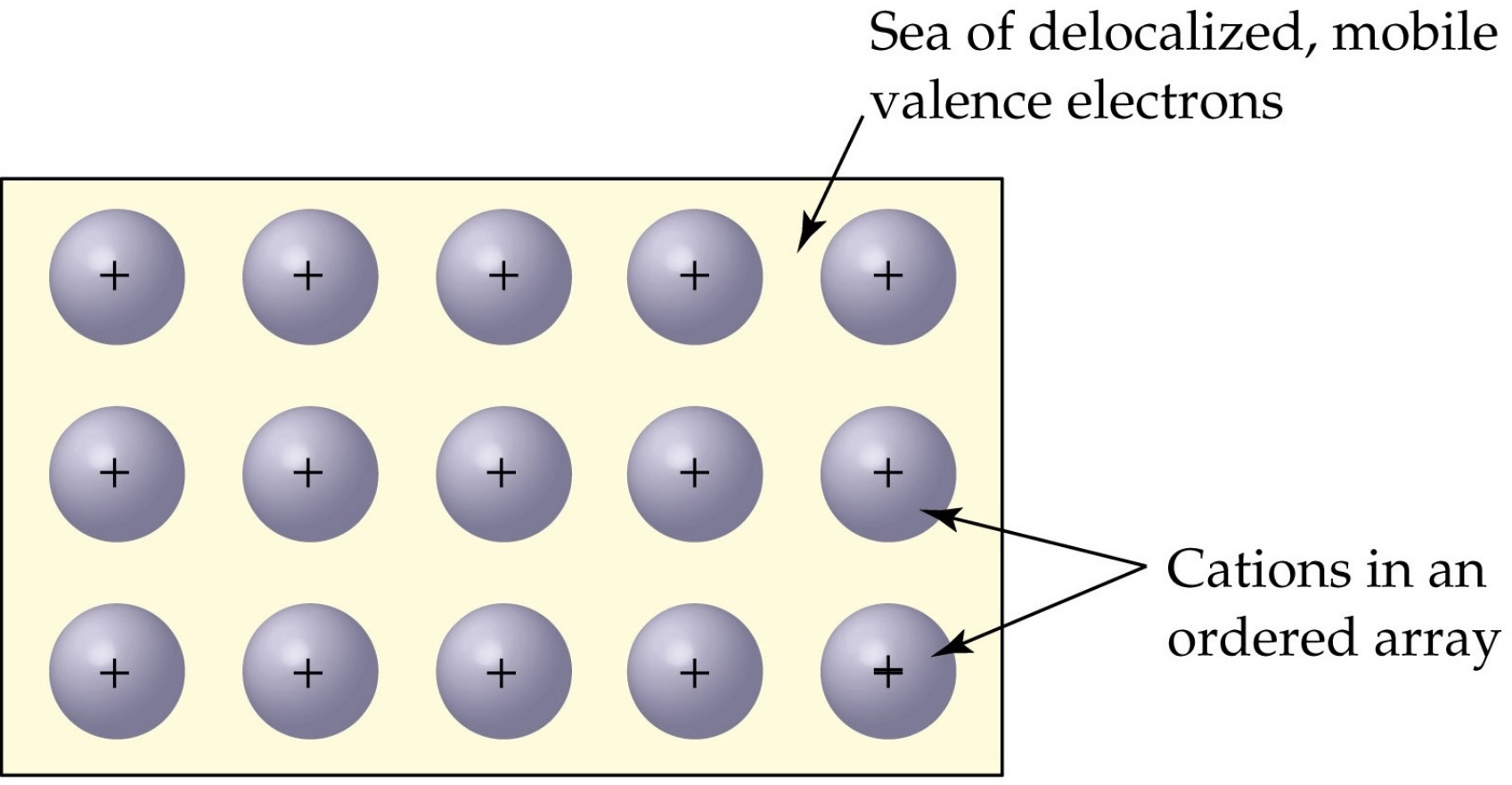
explanantion of sea of electrons model
metallic solids conduct electricity
because the free e- are mobile, it allows the electrons to move through the metallic crystal and conduct electricity
as temperature increases, electrical conductivity in metals decreases
heating causes the metal ions to vibreate faster, making it harder for electrons to make their way through the crystal
metals are malleable and ductile
because there are no localized bonds, the metal ‘cations’ can move past each other easily
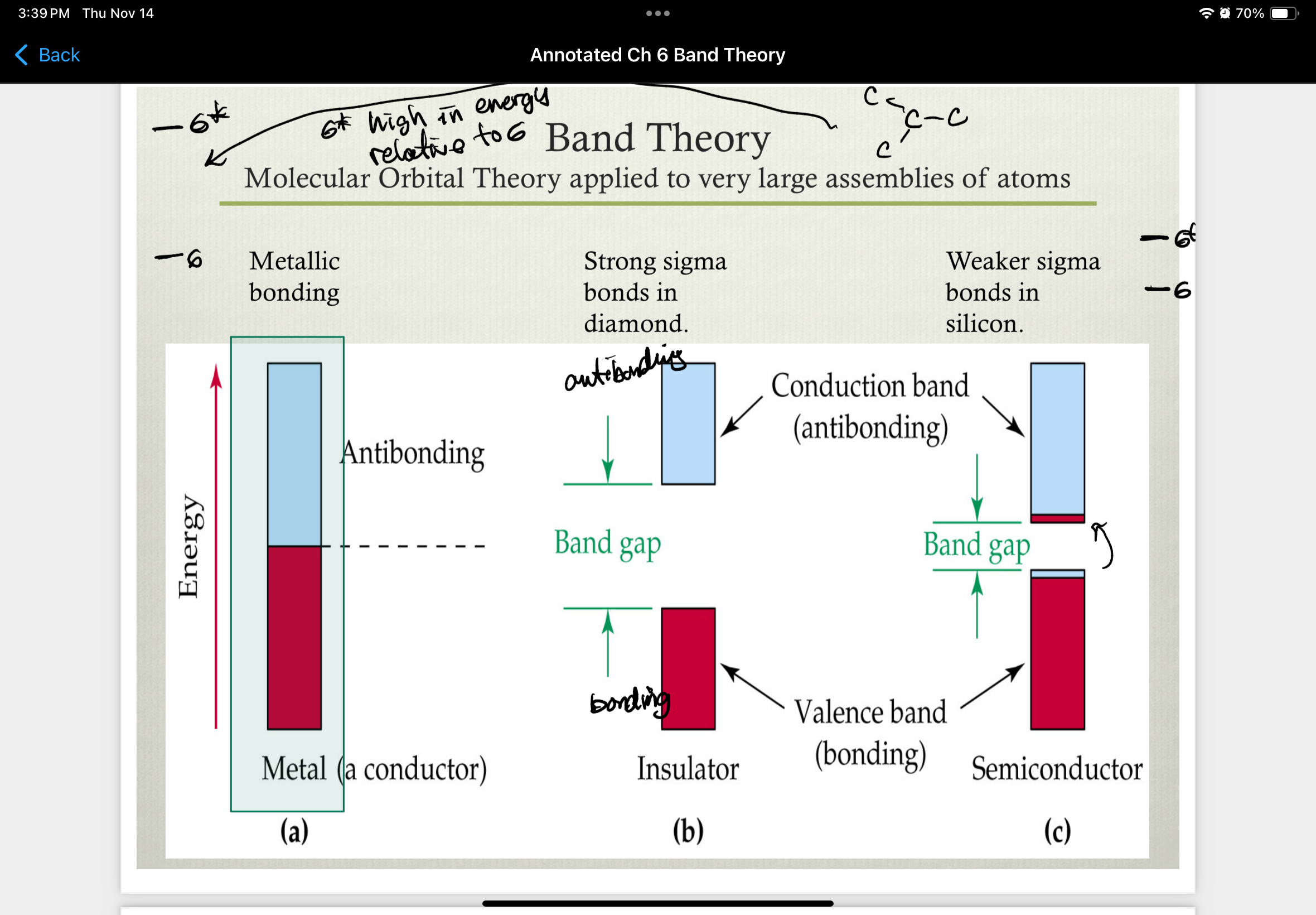
band theory (molecular orbital theory applied to very large assemblies of atoms)
as you add more and more molecular orbitals you get a “smear” of orbitals because of the Pauli Exclusion Principle
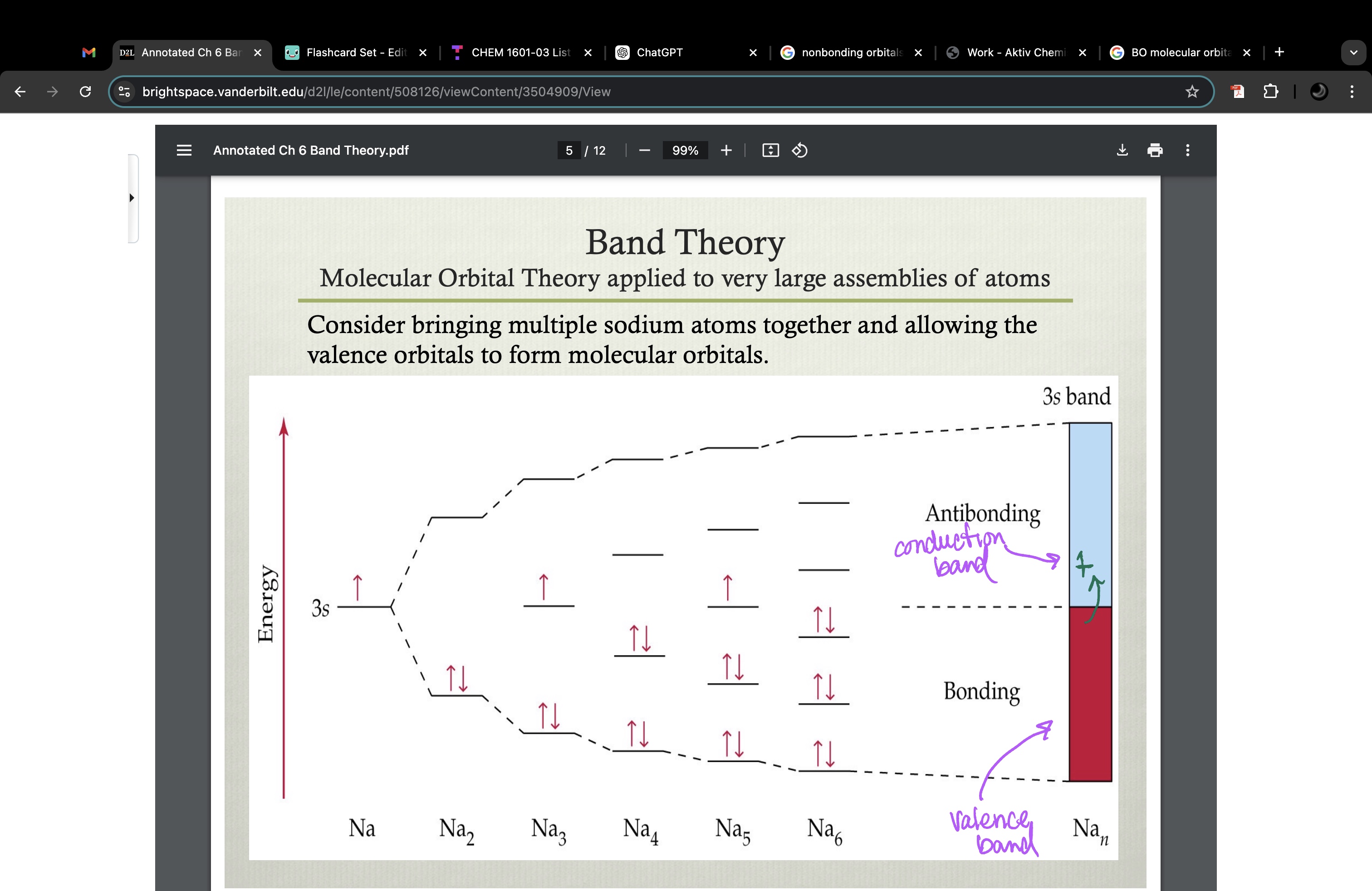
current (electron movement) occurs when electrons can move into empty orbitals in the conduction band
Pauli exclusion principle prevents an electron moving into a orbital that already has 2 electrons
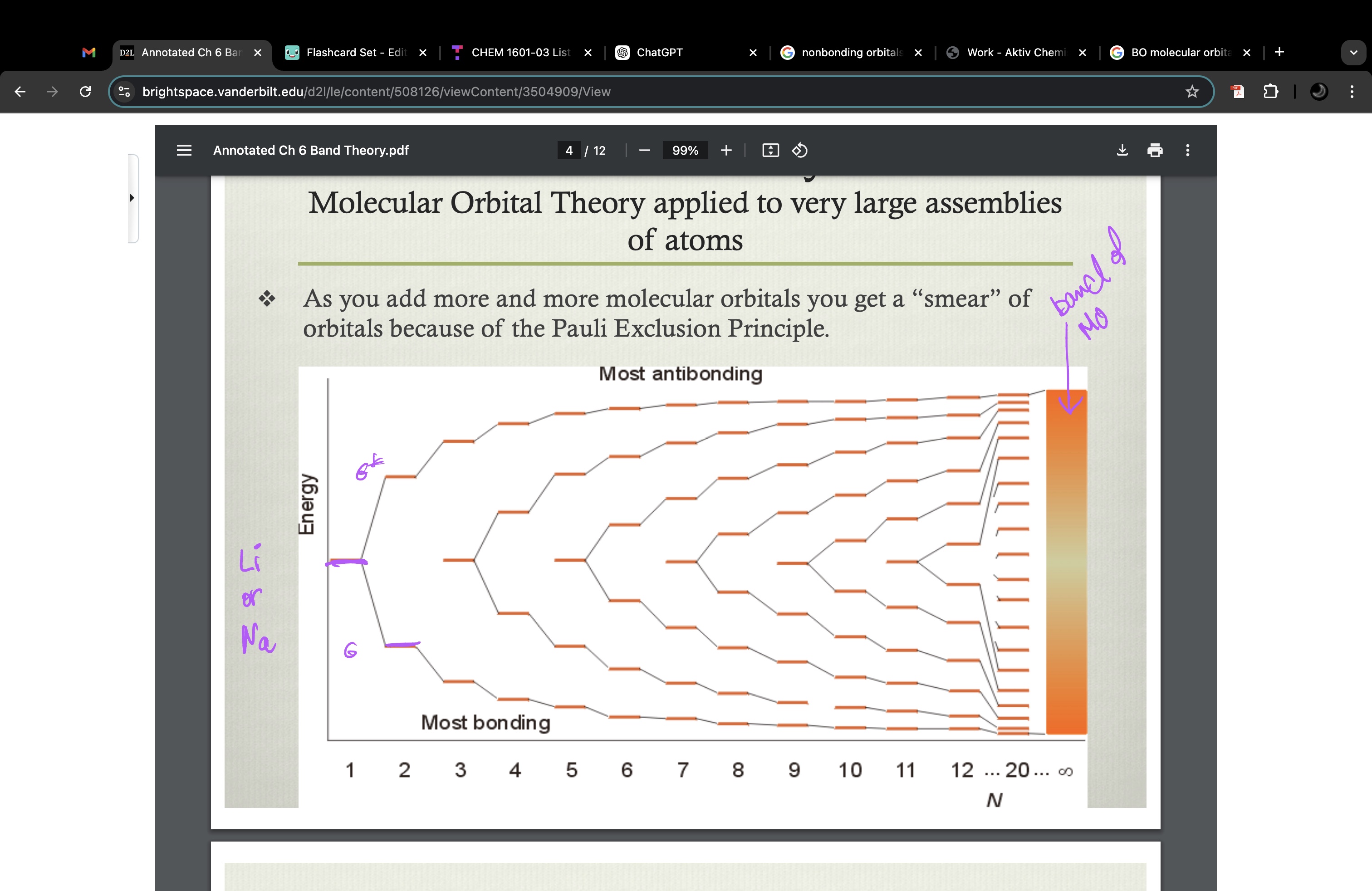
MO theory for metal electrical conductivity
adding atoms to create lattice structure: energy levels are so close together, it takes very little energy to excite an electron from the HOMO into the LUMO of sodium (and most metals). Once an electron is promoted to the LUMO, it can travel through the entire metal sample with little resistance since the LUMO is not occupied by other electrons (no e-/e- repulsion) and extends over the entire metal sample. This is the MO theory explanation for the electrical conductivity of metals!
ductility
the ability of a metal to be drawn into thin wires
malleability
the ability of metals to be pressed out of shape without breaking or cracking
band gap
energy required to promote an electron from the valence band to the conducting band
valence band = band occupied with e-
conducting band = unoccupied band
insulators
do not conduct electricity because there is a relatively large difference between the valance band and the conduction band
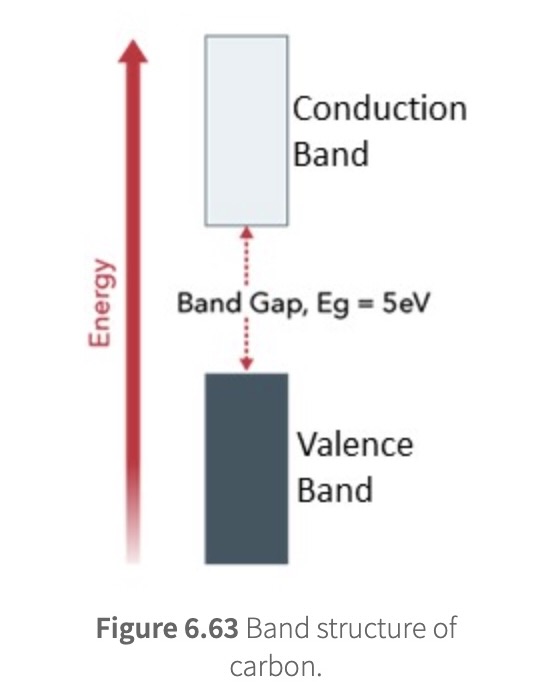
semiconductor
a material with an electrical conductivity falling between a conductor and an insulator, and a resistance that decreases as temperature increases (opposite of a metal)
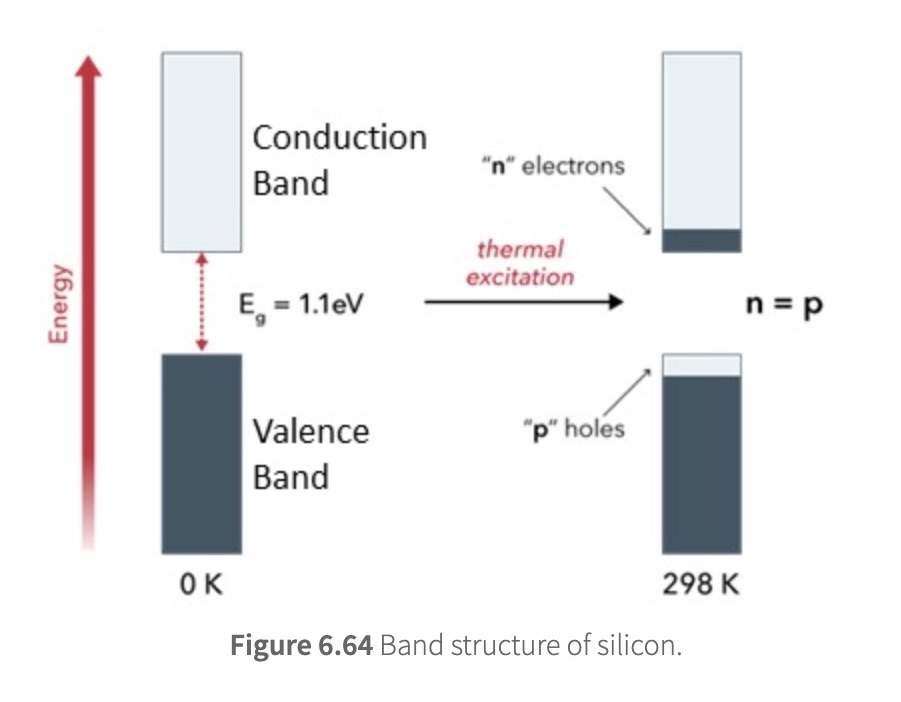
Silicon has the same number of valence electrons as carbon and has a similar band structure to carbon
Compared with carbon, however, the band gap in silicon is smaller (1.1 eV)
only thermal energy or photons from sunlight are required to promote electrons from the valence band to the conduction band
When this happens, the valence band has empty molecular orbitals (holes; p) and allows electrons to flow through, and the conduction band has extra electrons (denoted as n) to flow through its empty molecular orbitals. As a result, silicon is enabled to conduct electrical current
metal, insulator, and semiconductor
metal = thermal energy promotes electrons into the conductance band, producing high conductance
semiconductors = at room temp. only very few electrons have enough energy to cross the band gap. This results in low levels of conductance.
insulators = at room temp. essentially no electrons have enough energy to cross the band gap. NO conductance.
doping semiconductors
the process of adding an electron-rich or electron-poor element to an intrinsic semiconductor to tune the properties of the semiconductor
intrinsic semiconductor = pure material that behaves as a semiconductor
by adding impurities of specific atoms to silicon it is possible to change the effective band gap of the semiconductor
n-type doped semiconductors (electrons are the charge carriers)
semiconductors doped with electron-rich elements
group 5 (or 15) elements are added to Si because the group 5 atoms have one more valence electron
ex: Phosphorus
this electron sits in an orbital that doesn’t bond with the Si atoms around it
electrons move freely in conduction band, increasing conductivity
p-type doped semiconductors (holes are the charge carriers. holes are empty or partially empty orbitals in the valence band. because there is a missing electron at a “hole” the atom has a positive charge)
semiconductors doped with electron-poor elements
group 3 elements such as boron or galium are added to Si
the acceptor levels are right above the valence band so that electrons are easily promoted to the acceptor level, leaving holes in the valence band
atom with fewer than 4 e- will create a hole so there is no bond, hole shifts as other electrons try to fill the gap; creates + charge flow and boosts conductivity
conductors
energy gap between HOMO and LUMO is negligible
e- move freely throughout band