BIOS 352 - Exam 3
1/101
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
102 Terms
G (Gibbs free energy)
Amount of energy capable of doing work in a reaction
ΔG is negative when reaction releases free energy (exergonic)
ΔG is positive when reaction uses free energy (endergonic)
S (Entropy)
Measure of randomness or disorder
A gain of entropy means that products are less complex and more disordered than reactants
The entropy of the universe increases, but in a reacting system it can decrease
ΔGo
Standard free energy change, or ΔG in standard conditions
298K or 25oC
Reactants and products at 1M
Partial pressure of 1 atm
ΔG'o and K'eq
Standard transformed constants for biological reactions
pH of 7
Concentration of water at 55.5M
1mM of magnesium
Keq
equilibrium constant
[C]^c[D]^d/[A]^a[B]^b
(Products)/(Reactants)
ΔG'o = -RTlnK'eq
Standard ΔG is related to Keq
Where R = gas constant = 8.315J/molK = 0.008315kJ/molK
ΔG'o is an alternative way of expressing K'eq
ΔG = ΔG'o + RTln([C]c[D]d/[A]a[B]b) = ΔG'o + RTlnQ
The insides of cells rarely have standard conditions, so to relate ΔG to ΔG'o
Mass Action is Q - Amount of products and reactants determine whether the reaction can proceed
ΔG'o of sequential (coupled) reactions, additive or multiplicative?
They are additive
Endergonic reactions can proceed forward by coupling it to a highly exergonic reaction through a common intermediate
K'eq of sequential (coupled) reactions, additive or multiplicative?
Multiplicative
Oxidation
Loss of electrons
Reduction
Gain of electrons
Reducing Agents
Electron donors that get oxidized
Oxidizing Agents
Electron acceptors that get reduced
What are some examples of electron carriers?
NADPH and NADH
FMN and FAD
Lipid-soluble quinones in membranes
Iron-sulfur clusters and cytochromes
What are the four ways of transferring electrons?
Just as an electron
As a hydrogen atom (H+ and an electron)
As a hydride ion (hydrogen with two electrons)
In combination with O2
What is the order of most reduced to most oxidized for carbons?
Alkane - Alcohol - Aldehyde (Ketone) - Carboxylic Acid - Carbon Dioxide
NAD+
Nicotinamide adenine dinucleotide
Water-soluble co-enzyme involved with glycolysis
generally used in oxidations and catabolism
Two nucleotides are joined together by phosphate groups by a phosphoanhydride bond
When NAD+ is reduced (NADH), it has higher absorbance at >300nm
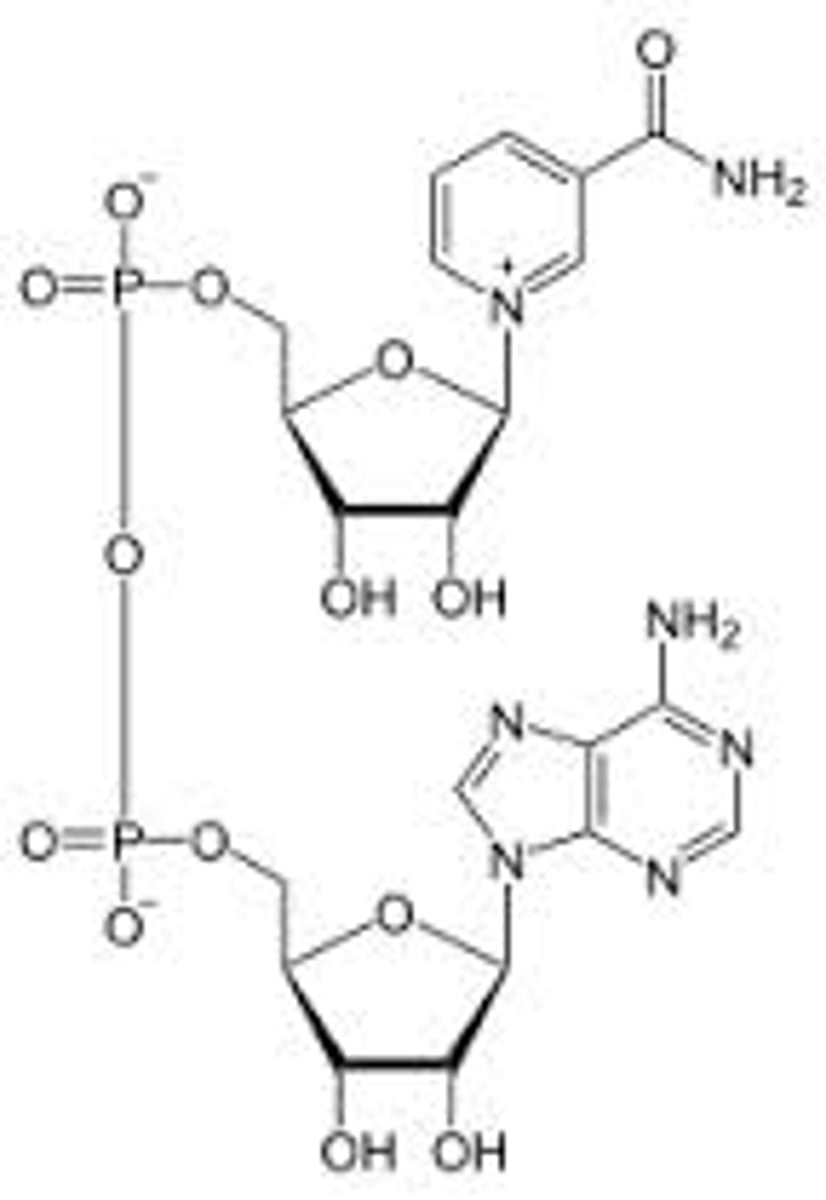
NADP+
Nicotinamide dinucleotide phosphate
Water-soluble co-enzyme involved with reductions and anabolism
A hydroxyl group is esterified with a phosphate
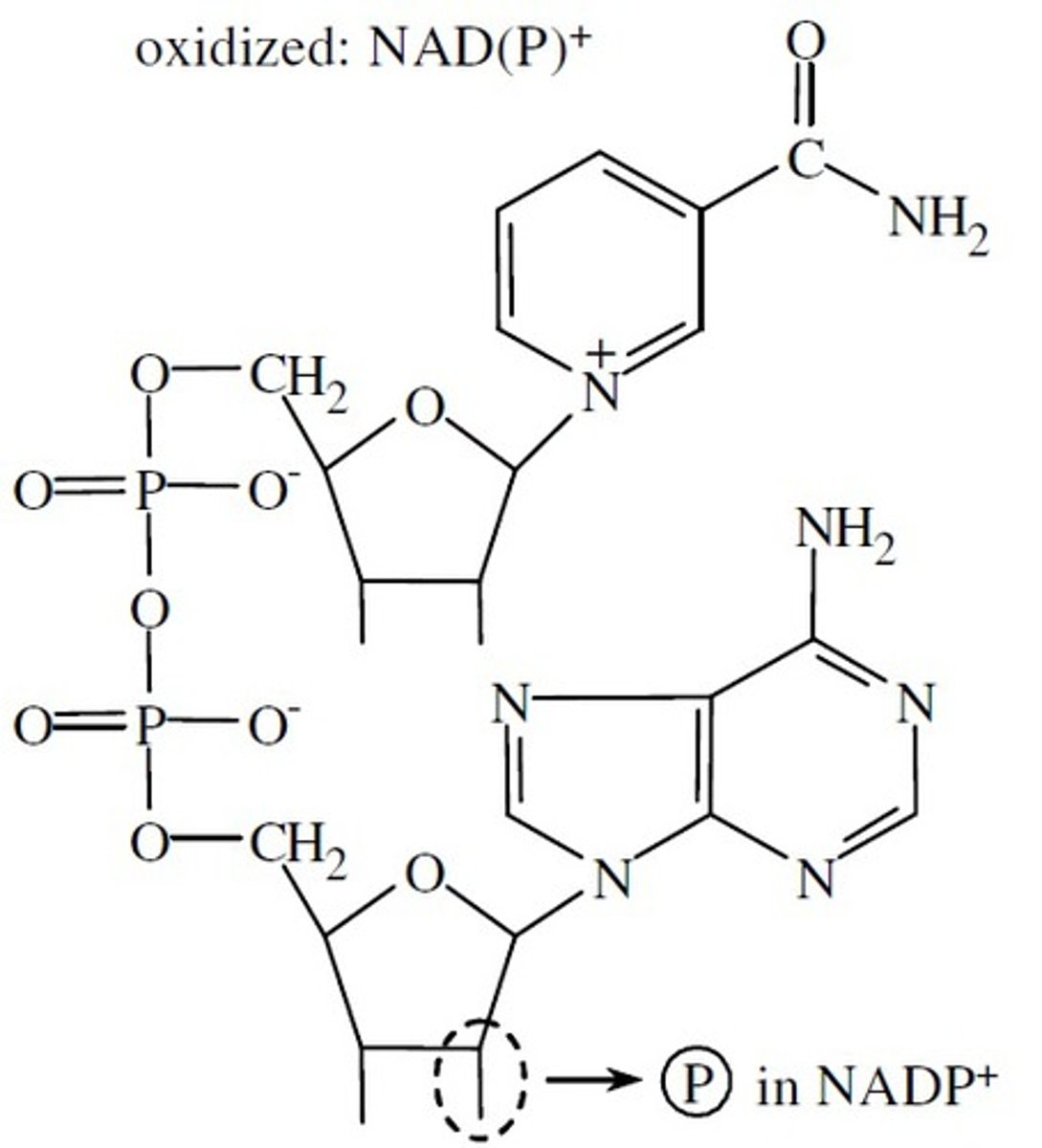
What happens to the nicotinamide ring when the substrate is oxidized?
The ring accepts a hydride ion and is reduced to NADH or NADPH
The second proton removed is released into the aqueous solvent
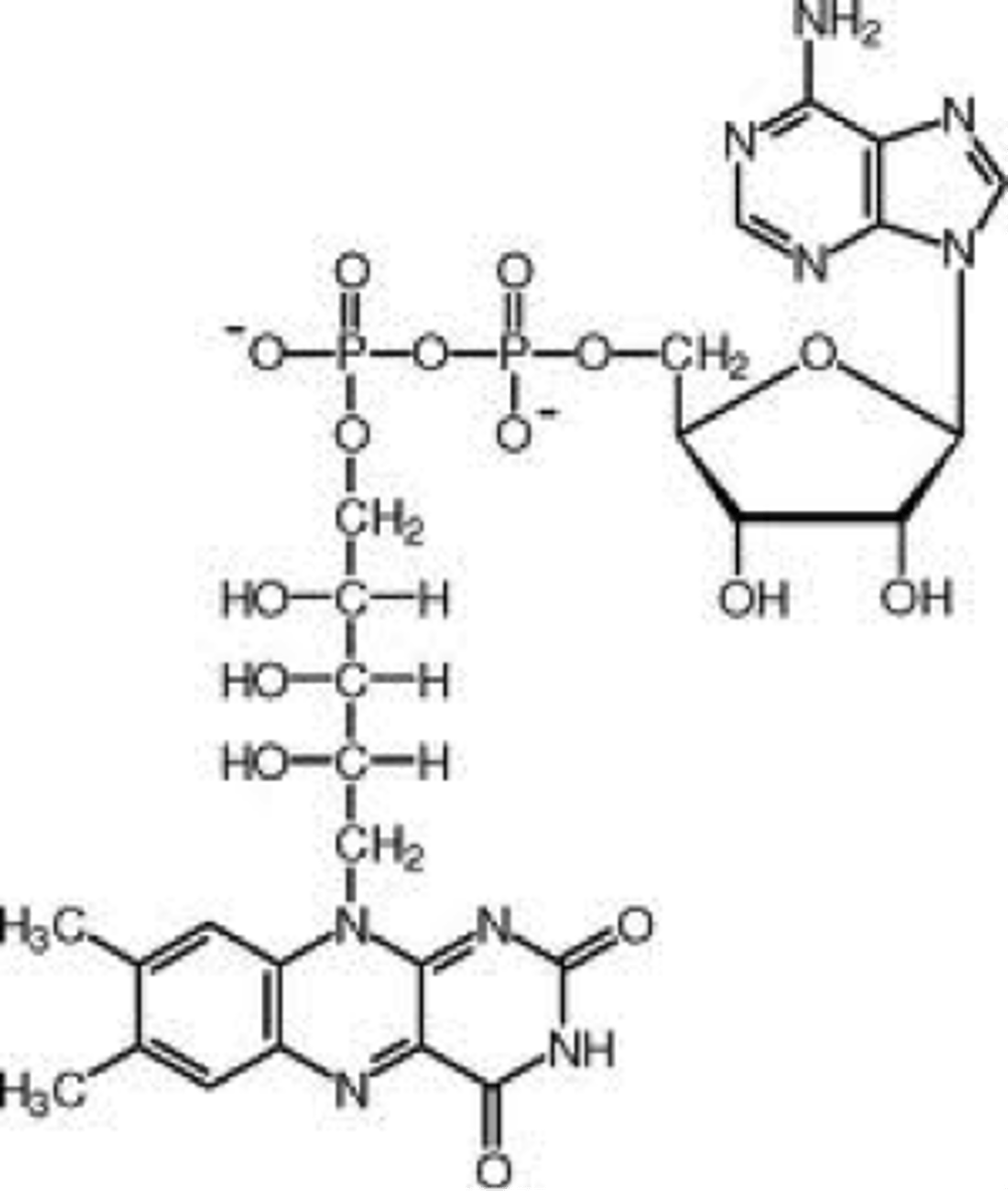
FMN
Flavin mononucleotide
derived from riboflavin (Vitamin B2)
Part of the entire FAD
Can undergo reduction w/ two hydrogen atoms (FMNH2) or one hydrogen (semi-quinone FMNH)
Tend to stay with an enzyme as a prosthetic group due to their covalent and tight binding (flavoproteins)
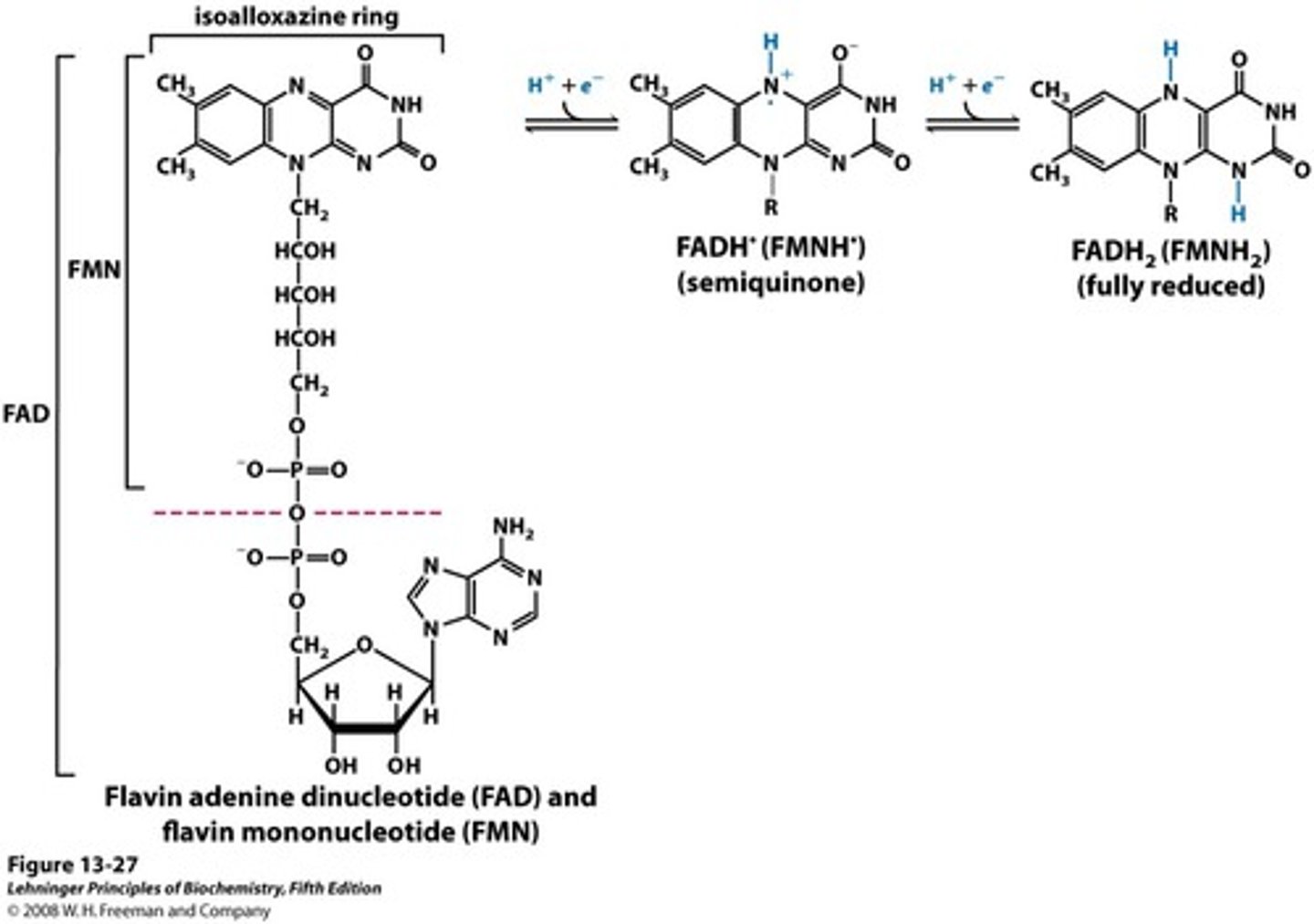
FAD
Flavin adenine dinucleotide
derived from riboflavin (Vitamin B2)
Can undergo reduction w/ two hydrogen atoms (FADH2) or one hydrogen (semi-quinone FADH)
Tend to stay with an enzyme as a prosthetic group due to their covalent and tight binding (flavoproteins)
What are the products of glycolysis?
2 pyruvate
2 NET ATP
2 NADH
What are the two phases of glycolysis?
preparatory phase (consumed 2 atp) and payoff phase (produce 4 atp, 2 pyruvate, and 2 NADH)
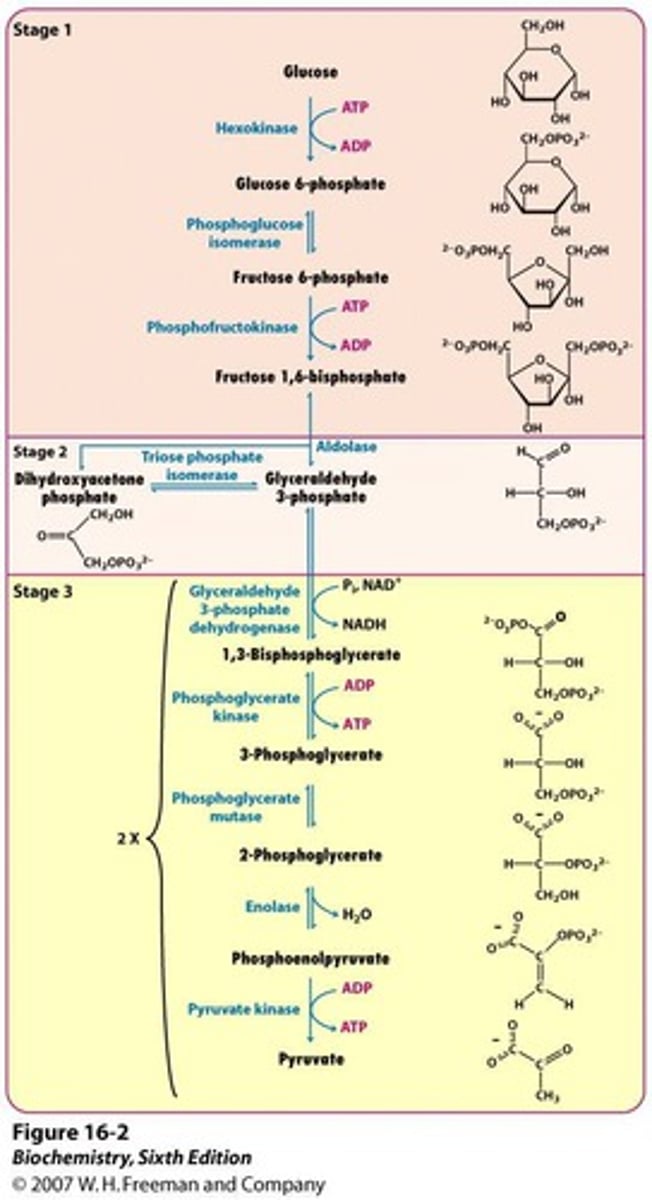
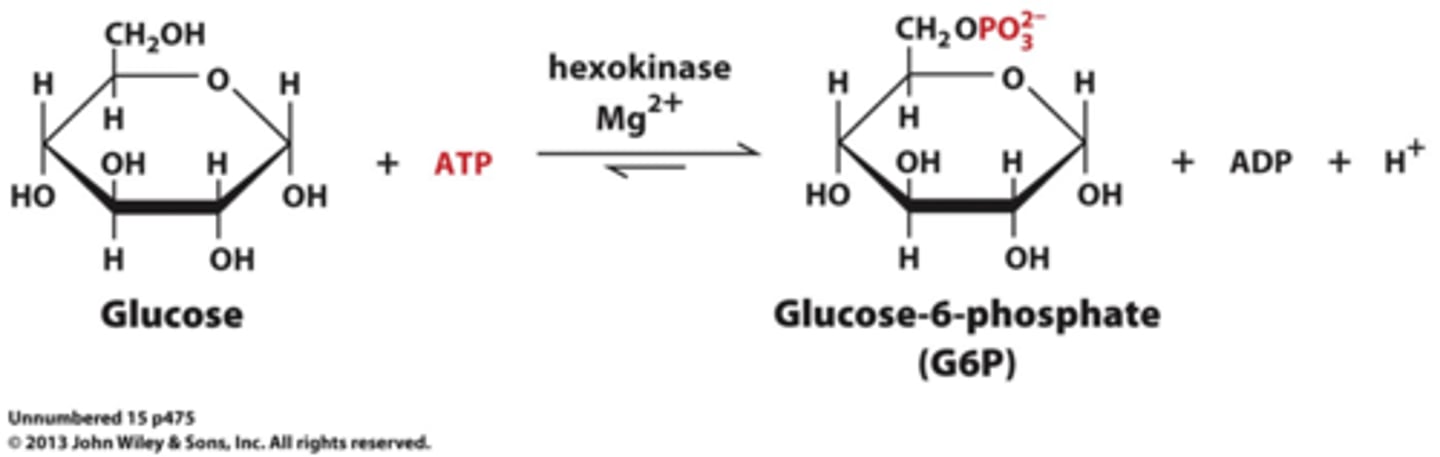
What is the first step of glycolysis?
The Hexokinase Reaction (Glucose to Glucose 6-Phosphate)
Phosphorylation is catalyzed by hexokinase in eukaryotes, and glucokinase in prokaryotes
Nucleophilic oxygen at C6 of glucose attacks the last (γ) phosphorous of ATP
ATP provides the phosphoryl group
Bound Mg2+ facilitates this process by stabilizing the negative charge in the transition state
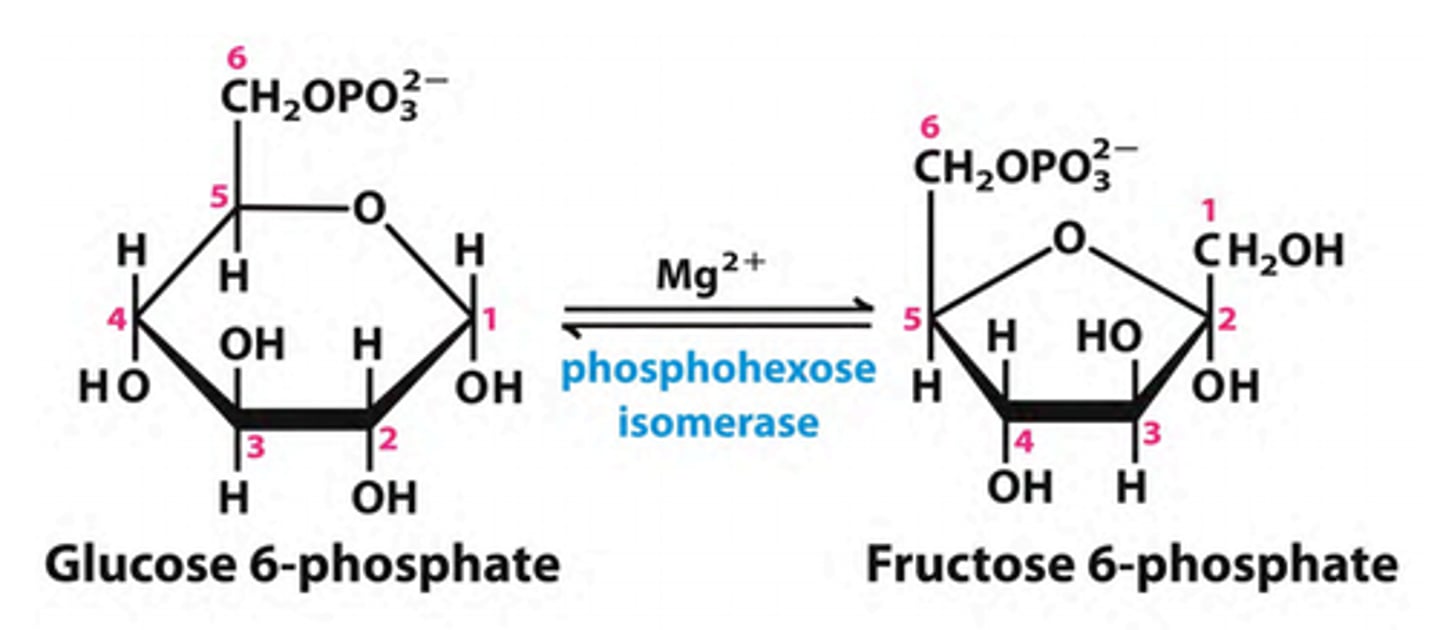
What is the second step of glycolysis?
Phosphohexose Isomerization (Glucose 6-phosphate to Fructose 6-phosphate)
An aldose can isomerize into ketose
Isomerase - Enzyme that can change the general structure of a molecule without changing the molecular structure
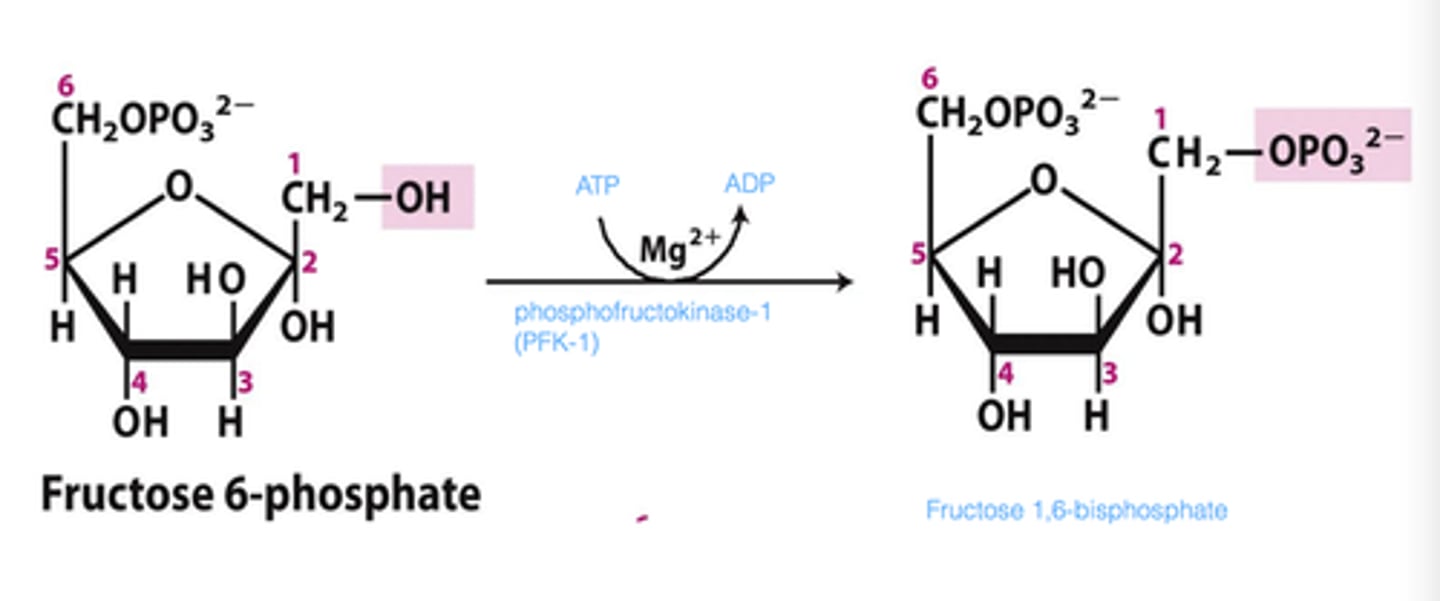
What is the third step of glycolysis?
The Priming Reaction (Fructose 6-phosphate to Fructose 1,6-bisphosphate)
The First Commitment - This step is irreversible!
Another ATP provides a phosphoryl group
The Fructose 1,6-bisphosphate is now committed to becoming pyruvate and yielding energy
Phosphofructokinase-1 (the enzyme) is negatively regulated by ATP
This means that if there isn't a lot of ATP, the enzyme will work harder to catch up
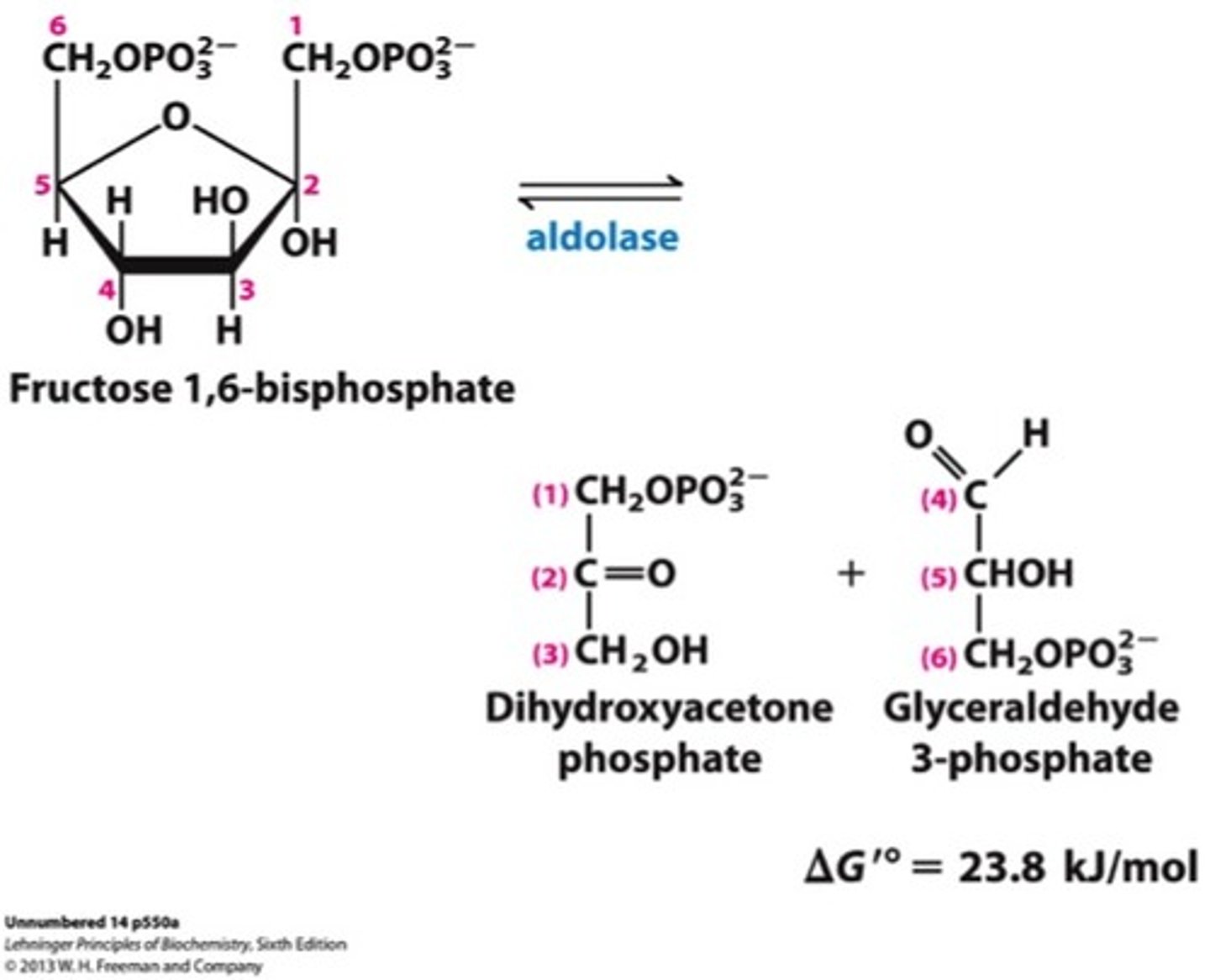
What is the fourth step of glycolysis?
Aldolases cleave 6-carbon sugars (Fructose 1,6-bisphosphate to dihydroxyacetone phosphate and glyceraldehyde 3-phosphate)
Uses aldolase
The reverse process of this is aldol condensation
Last step of the preparatory phase!
What is the fifth step of glycolysis?
Triose phosphate interconversion (Dihydroxyacetone to glyceraldehyde 3-phosphate)
Aldolase cleaves the fructose into two triose phosphates: DAP and GAP
GAP is the only substrate for the next enzyme, so DAP is converted enzymatically (via triose phosphate isomerase) to GAP
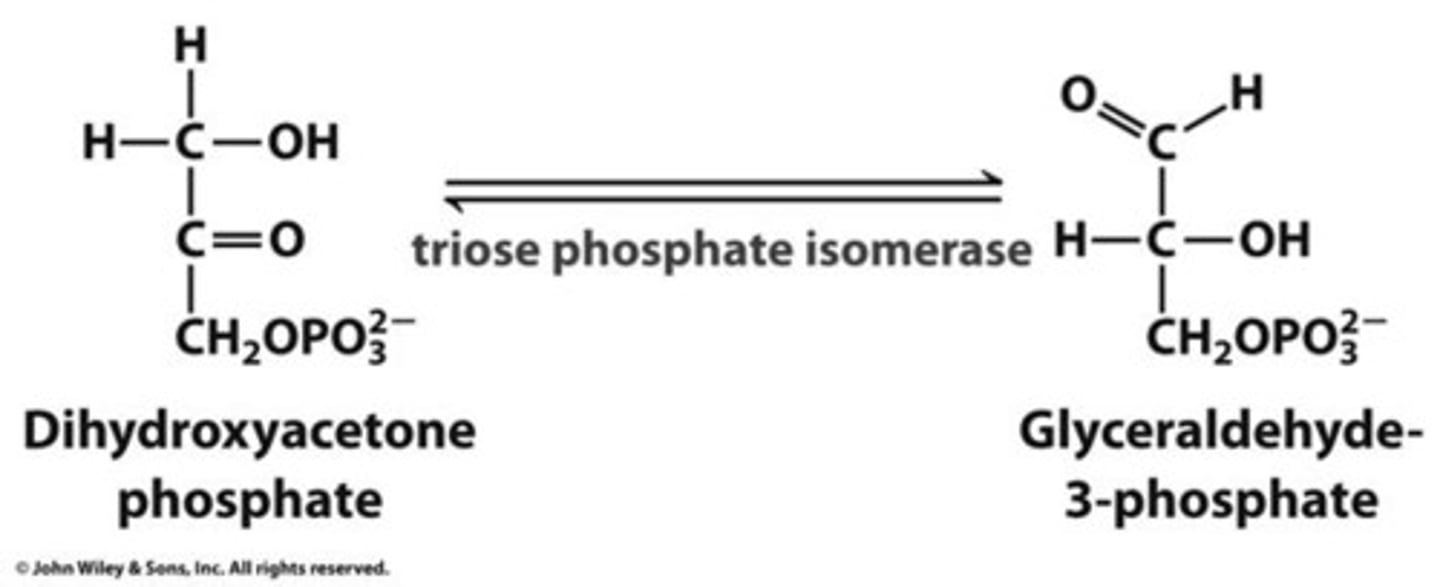
Where are the carbons after step 5?
Glucose carbons 1-3 are on DAP, 4-6 are on GAP
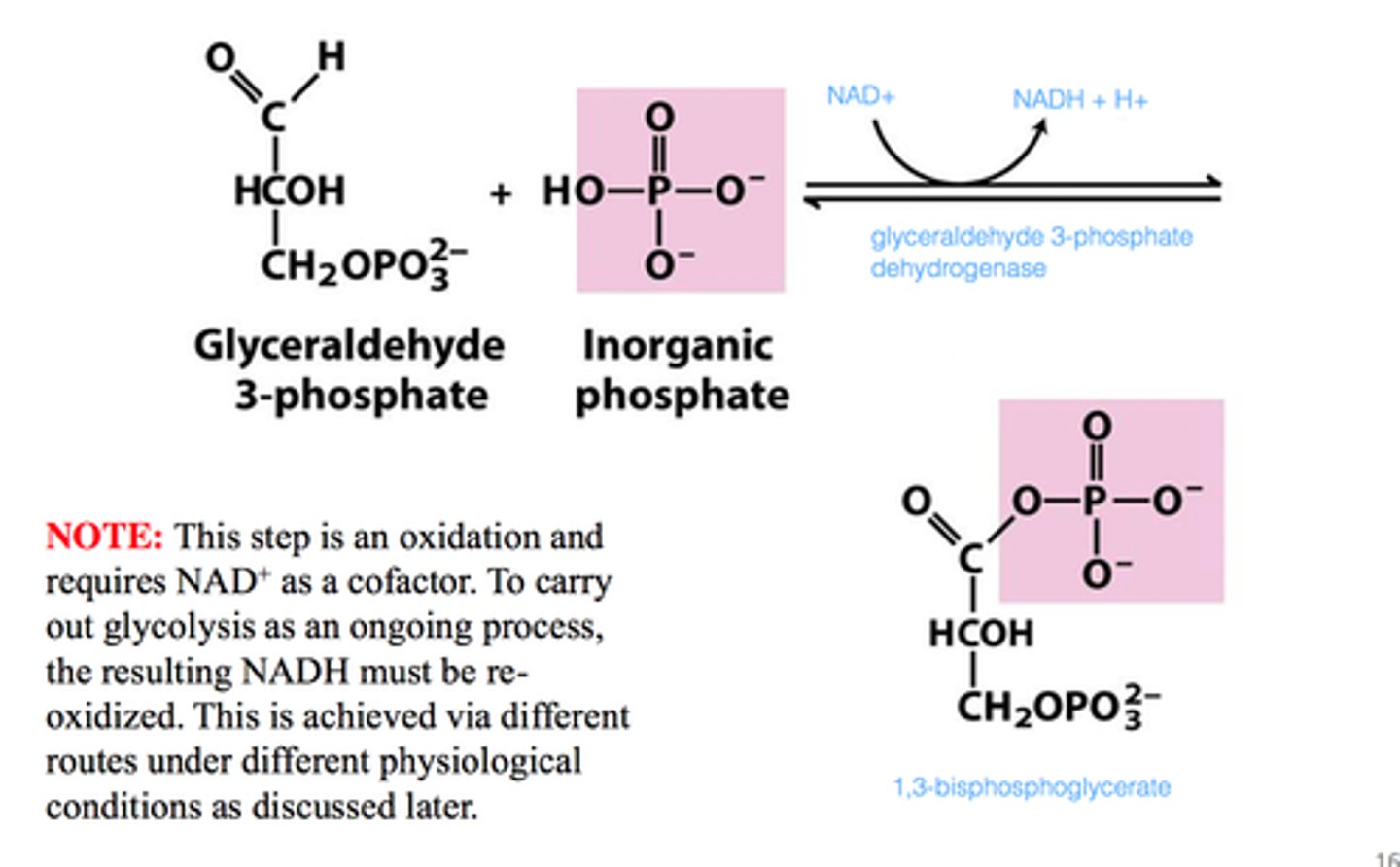
What is the sixth step of glycolysis?
G3P dehydrogenase reaction (Glyceraldehyde 3-phosphate to 1,3-Bisphosphoglycerate
The is the first energy-yielding step
The oxidation of aldehyde with NAD+ produces NADH
Phosphorylation yields a high-energy reaction product
The phosphoryl group comes from inorganic phosphate
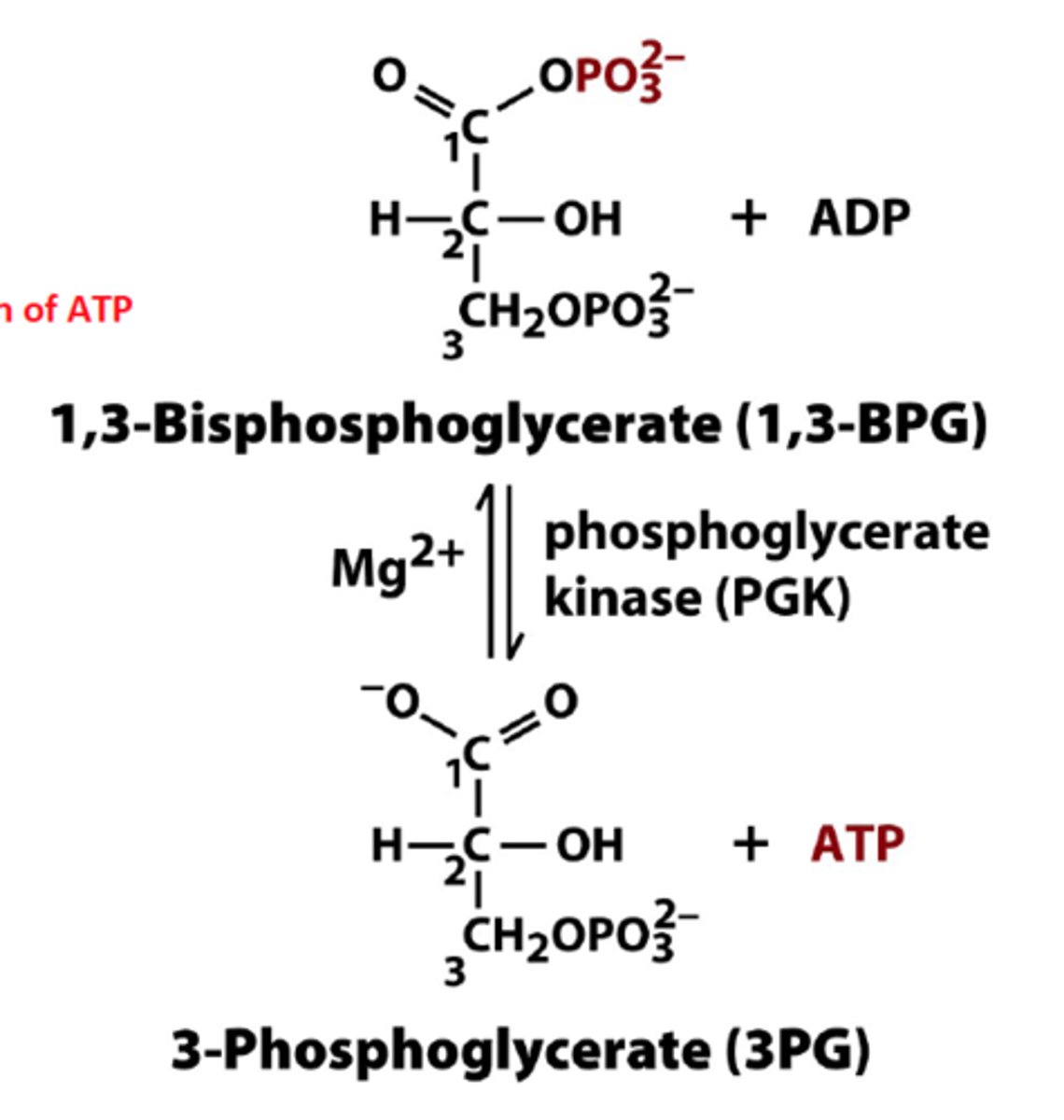
What is the seventh step of glycolysis?
First Substrate-Level Phosphorylation (1,3-Bisphosphoglycerate to 3-Phosphoglycerate
1,3-Bisphosphoglycerate is a high-energy compound that can donate a phosphate group to ADP to form ATP
The reaction is reversible, where the reverse process is the transfer of phosphate from ATP to phosphoglycerate
Kinases transfers phosphate groups from molecules
What is the eighth step of glycolysis?
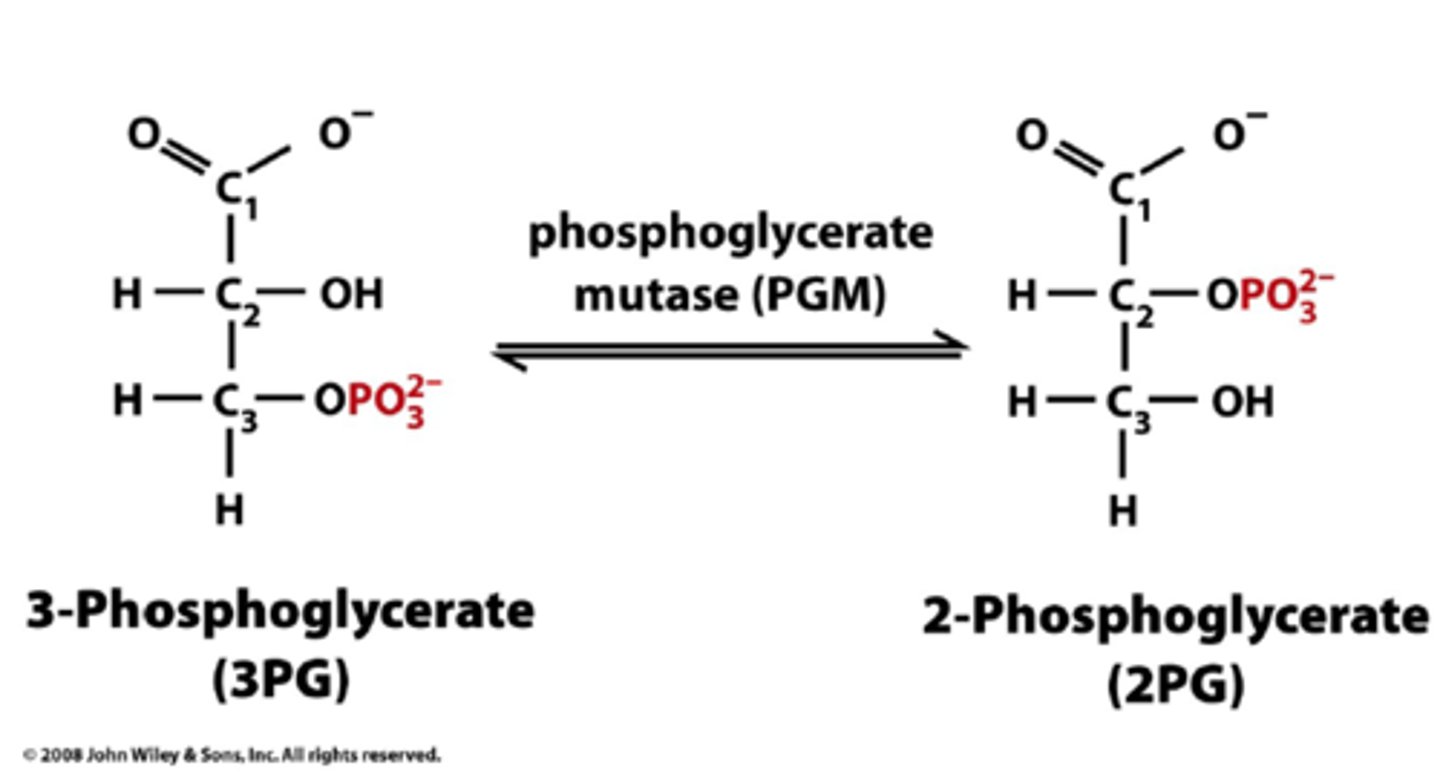
Conversion of 3-Phosphoglycerate to 2-Phosphoglycerate
Phosphoglycerate mutase
Reversible isomerization reaction
Mutase - Catalyzes movement of a functional group from one molecule to another
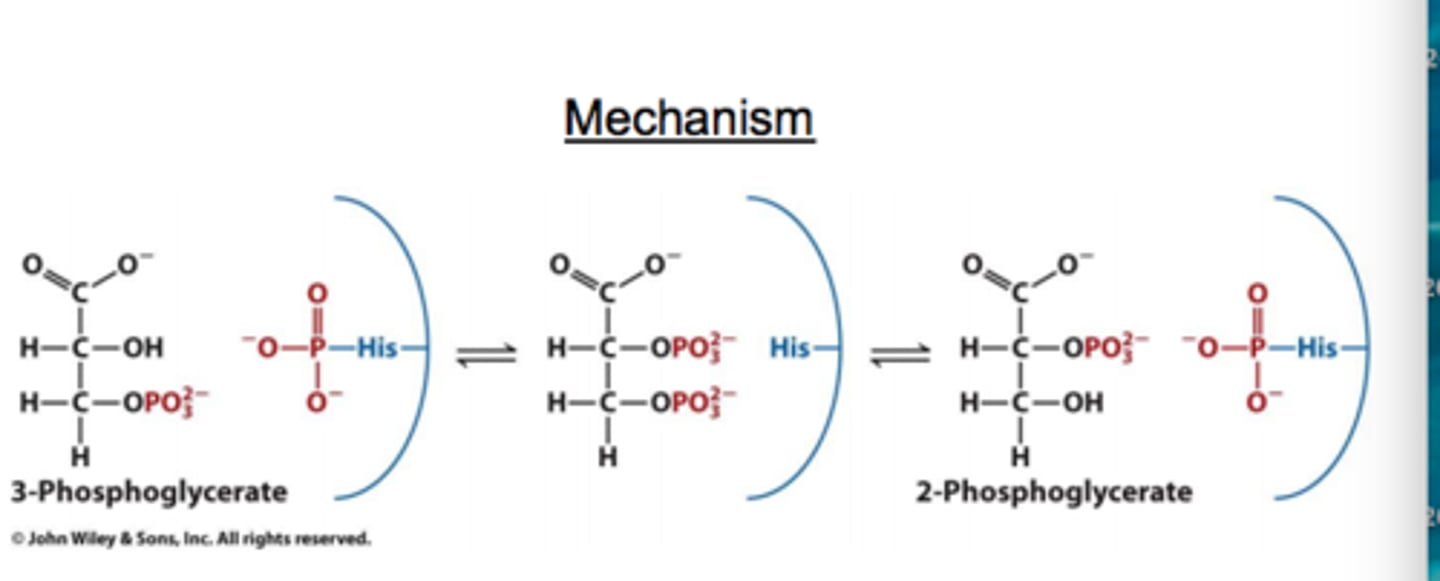
What is the mechanism of phosphoglycerate mutase reaction?
Uses covalent catalysis
Histamines are a part of the active site
Although the molecule now has two phosphate groups and negative charges close to each other, the 2-phosphoglycerate is not a good phosphate donor so the 3-phosphoglycerate donates instead
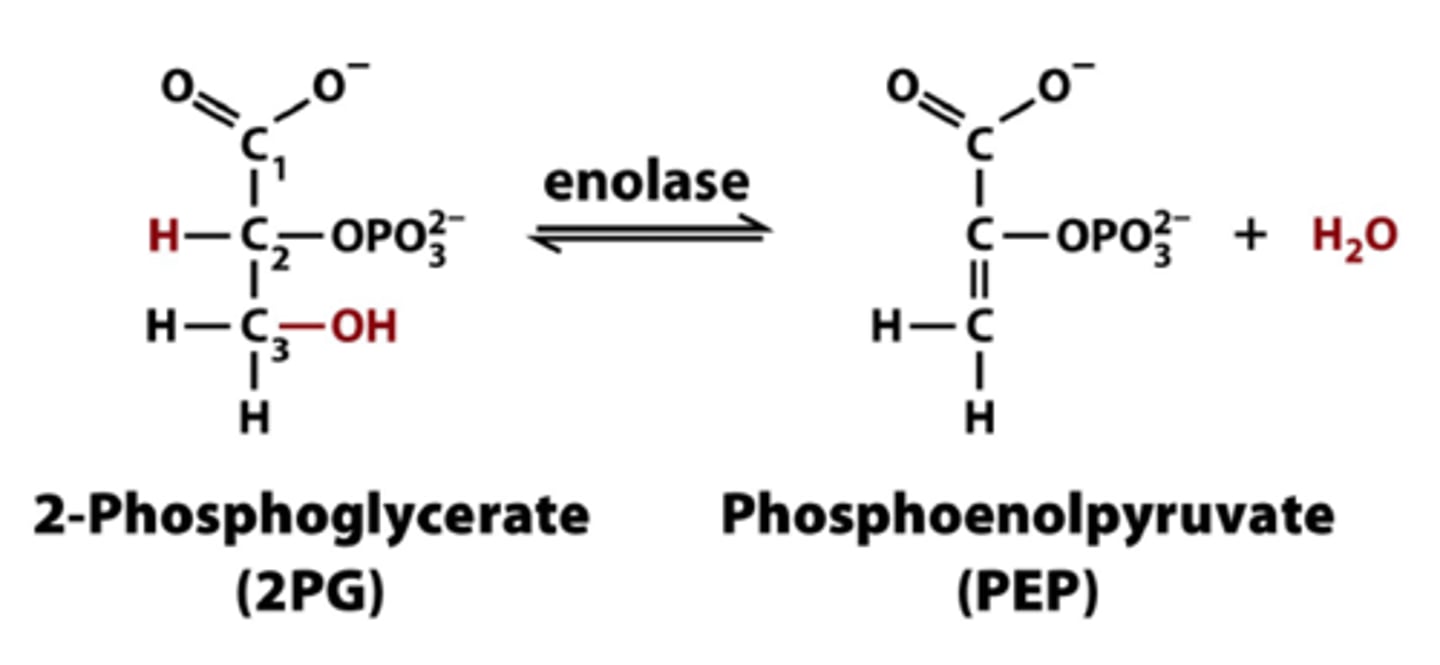
What is the ninth step of glycolysis?
Dehydration of 2-phosphoglycerate (2-phosphoglycerate to phosphoenolpyruvate)
Try to create a better phosphoryl donor
The loss of phosphate from 2-phosphoglycerate only creates a secondary alcohol with no stabilization, so phosphoenolpyruvate is used
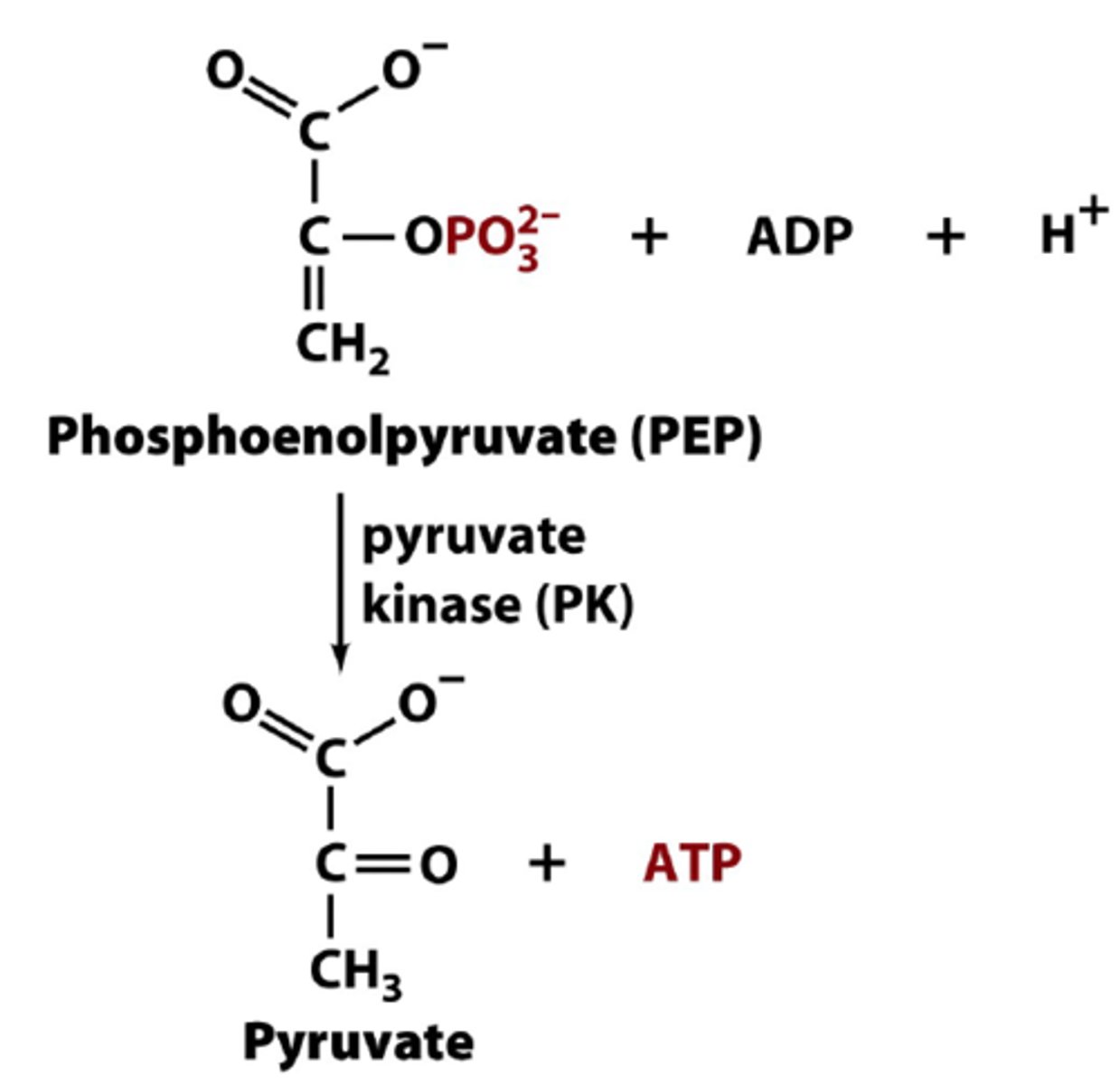
What is the tenth step of glycolysis?
Second substrate-level phosphorylation (Phosphoenolpyruvate to Pyruvate)
Produces another two ATPs
Phosphoryl group comes from phosphoenolpyruvate
The loss of phosphate from phosphoenolpyruvate yields an enol that tautomerizes into a ketone, however, this tautomerization effectively lowers the concentration of the reaction product, and drives the reaction towards ATP formation
What is the overall reaction of glycolysis and energy gain?
Glucose + 2NAD+ -> 2 pyruvate + 2NADH + 2H+ -> ΔG1'o = -146kJ/mol
2ADP + 2Pi -> 2ATP + 2H2O -> ΔG2'o = 2(30.5kJ/mol) = 61kJ/mol
ΔGtotal'o = -146kJ/mol + 61kJ/mol = -85kJ/mol
What is the feeder pathway for sucrose?
Can be split using sucrase to create glucose (which feeds from the beginning of glycolysis via hexokinase and ATP) and fructose (which can feed into the second step of glycolysis via hexokinase and ATP to form fructose 6-phosphate)
Alternatively, the fructose can be catalyzed by fructokinase and ATP to form fructose 1-phosphate, which is split into glyceraldehyde and DAP via fructose 1-phosphate aldolase. The glyceraldehyde is converted into glyceraldehyde 3-phosphate via ATP and triose kinase, and DAP is converted into glyceraldehyde 3-phosphate using triose phosphate isomerase.
What is the feeder pathway for Trehalose?
Can broken down into glucose using trehalase
What is the feeder pathway for mannose?
Broken down into mannose 6-phosphate via ATP and hexokinase, and then into fructose 6-phosphate via phosphomannose isomerase.
What is the feeder pathway for glycogen?
Can either be converted into glucose via a-amylase and water OR
Converted to glucose 1-phosphate via phosphorylase and an inorganic phosphorus, which is then converted to glucose 6-phosphate using phosphoglucomutase
What is the feeder pathway for lactose/galactose?
Lactose is broken down into glucose (which we already know the pathway for) and galactose, using lactase. The galactose is then converted to UDP-galactose, the UDP-glucose, and then lastly Glucose 1-phosphate
What do animals do in anaerobic conditions?
Reduce pyruvate to lactose
This acidification of muscle tissue prevents it from continuing strenuous work
The lactate can be transported to the liver and converted into glucose
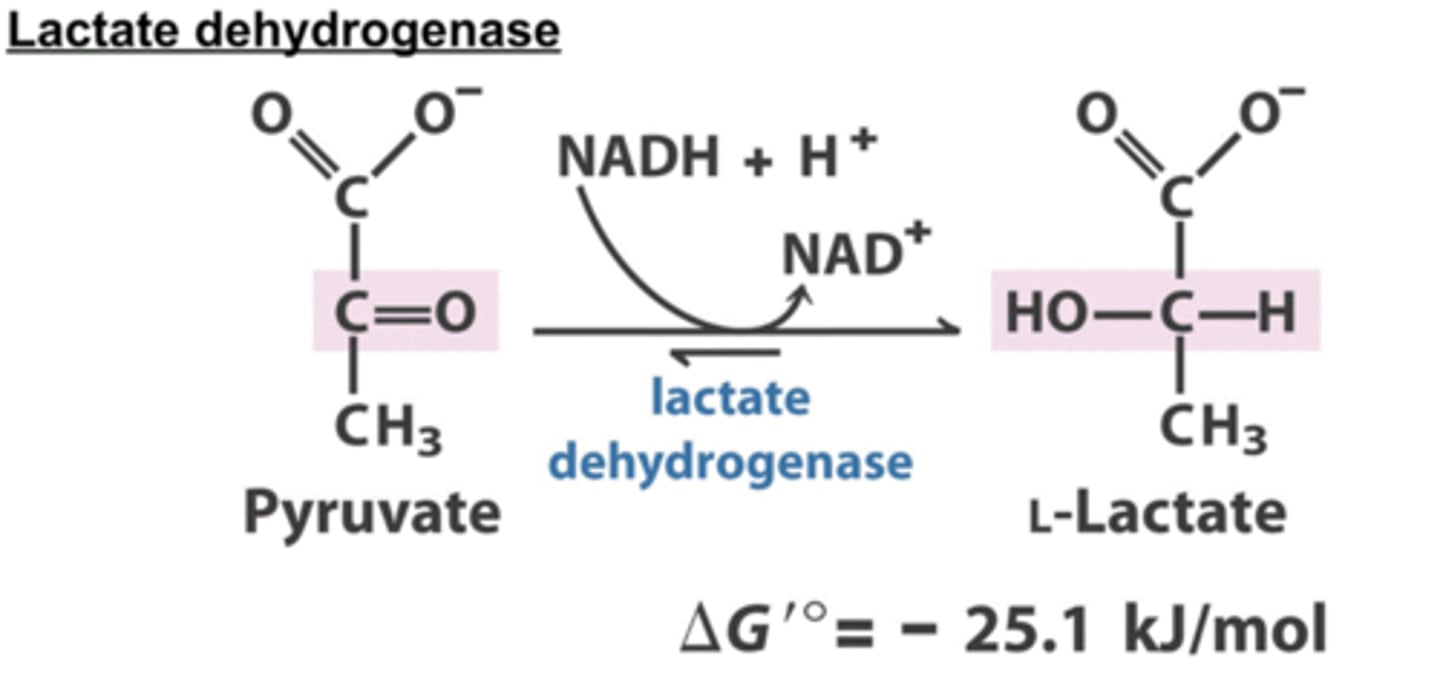
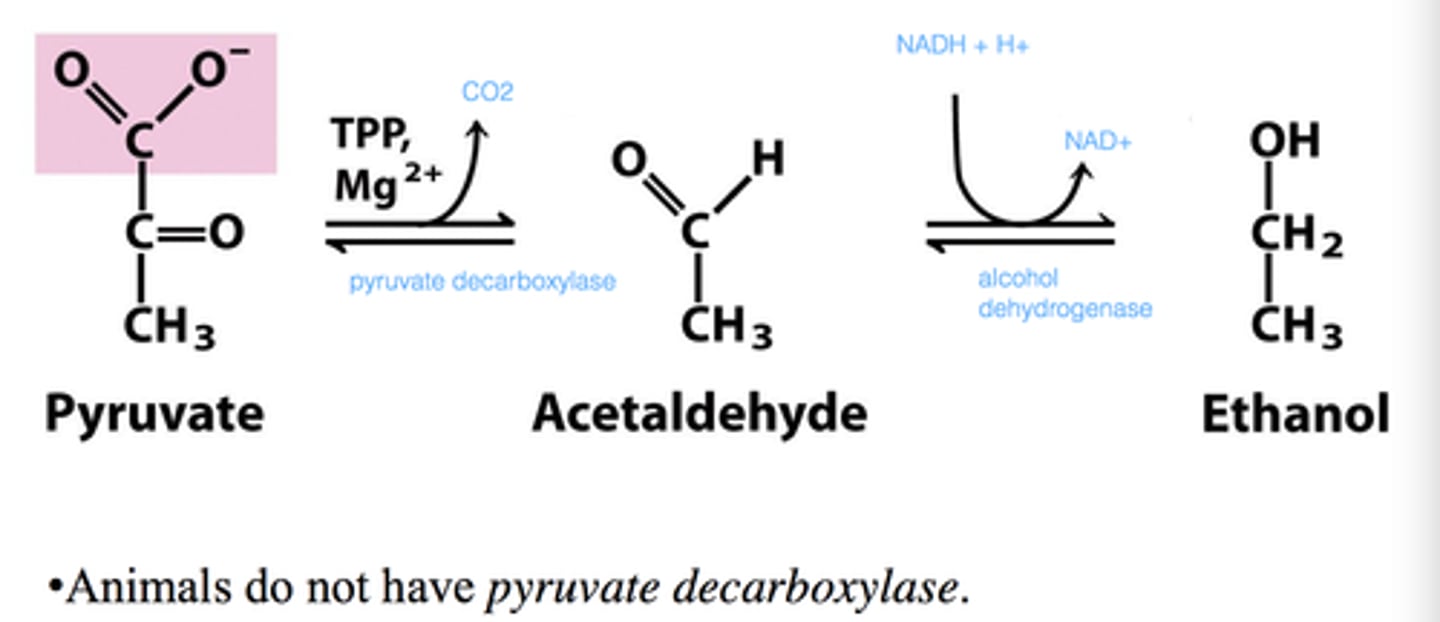
What does yeast do in anaerobic conditions?
ferments glucose to ethanol
Step 1: Pyruvate to acetaldehyde
Pyruvate decarboxylase, thiamine pyrophosphate (TPP) and Mg++ release CO2 to form acetaldehyde
Step 2: Acetaldehyde to ethanol
Alcohol dehydrogenase, Zn++, and NAD+ form ethanol
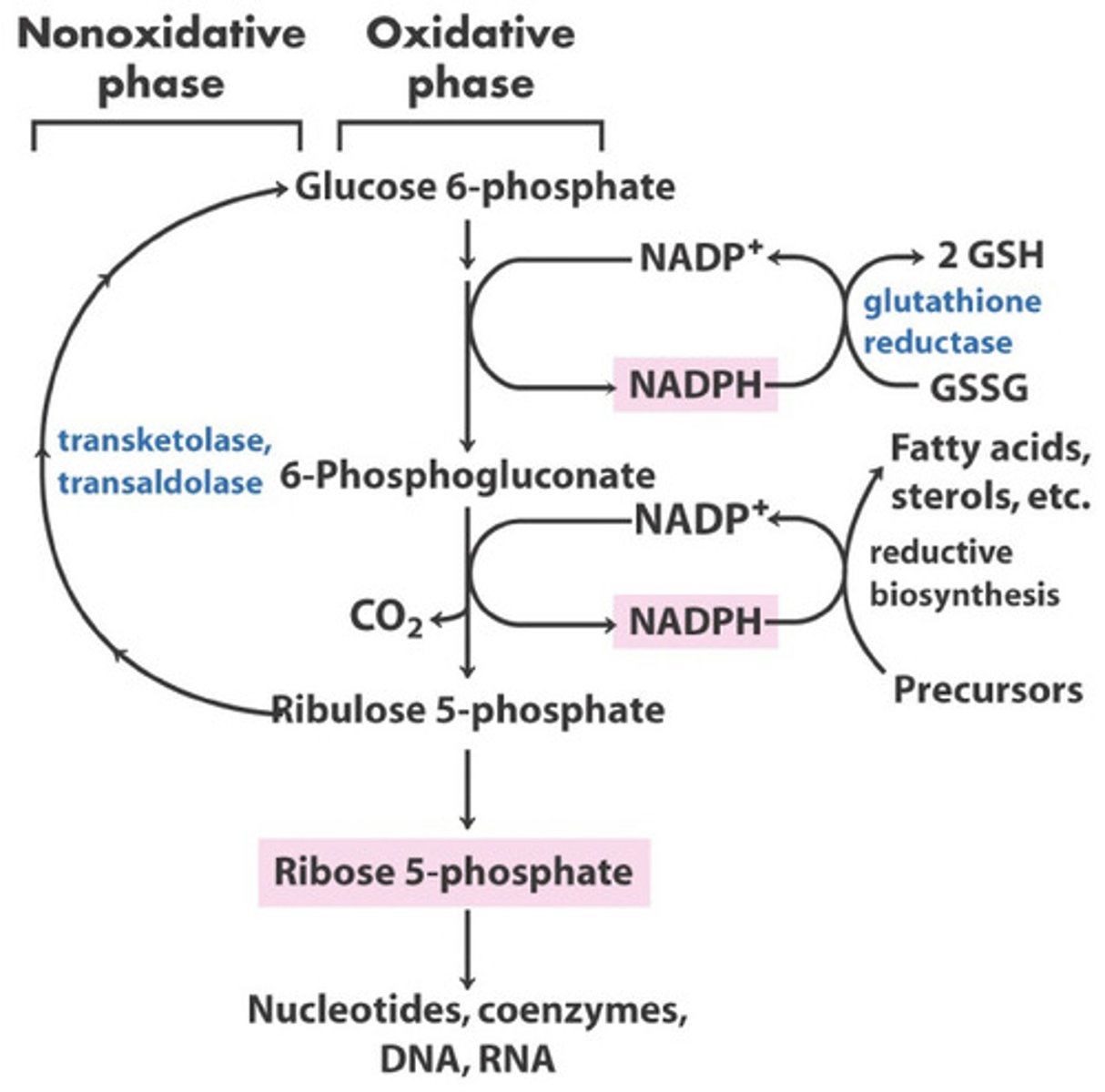
What is the purpose of the pentose phosphate pathway?
The main goals of the pentose phosphate pathway are to produce NADPH for anabolic reactions, and ribose 5-phosphate for nucleotides
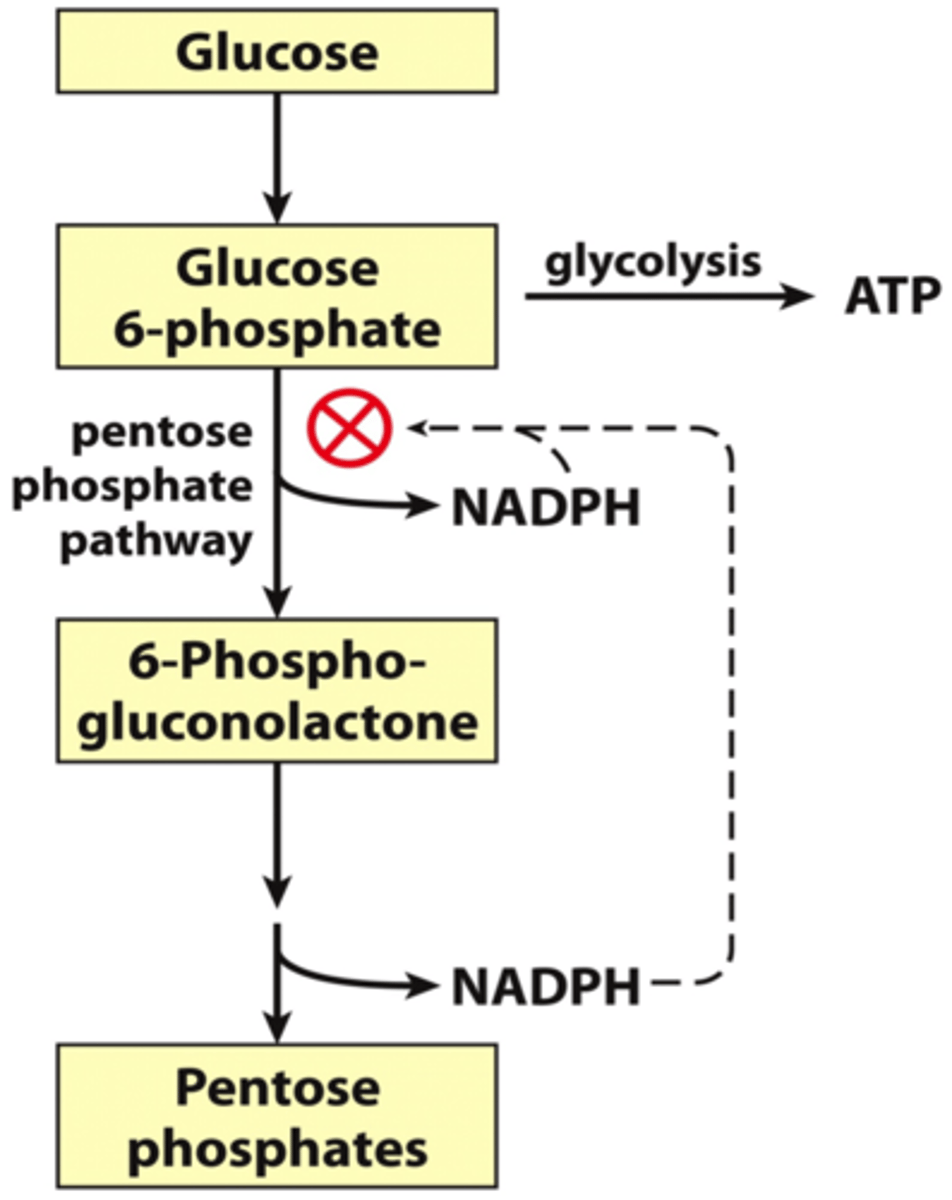
How is glycolysis and pentose phosphate pathway partitioned?
NADPH regulates partitioning
Inhibits glucose-6-phosphate dehydrogenase, which blocks the pentose phosphate pathway
What is gluconeogenesis?
Transforms smaller molecules, like lactate, pyruvate, glucogenic amino acids, triacylglycerols, glycerol, CO2 fixation and 3-phospho-glycerate into carbohydrates
What's the difference between glycolysis and gluconeogenesis?
Glycolysis occurs primarily in the muscle and brain (because they need a lot of energy) vs Gluconeogenesis occurs primarily in the liver
Glucose-6-phosphatase instead of hexokinase, and fructose 1,6-bisphosphatase-1 instead of phospho-fructokinase-1
Furthermore, the conversion of pyruvate to phosphoenolpyruvate requires two energy-consuming steps
ATP to ADP using pyruvate carboxylase, and GTP to GDP using PEP carboxykinase
What factors can change the rate of biochemical reactions?
Concentration of reactants
Level of activity of the catalyst
Concentration and intrinsic activity of the enzyme
Concentration of effectors
Allosteric regulators
Competing substrates
pH, ionic environment
Temperature
How does phosphorylation of enzymes affect their activity?
Protein phosphorylation is catalyzed by protein kinases
Dephosphorylation is spontaneous, or catalyzed by phosphoprotein phosphatase
Typically, hydroxyl groups of serine, threonine, and tyrosine are phosphorylated
What is an example of feedback inhibition?
ATP inhibiting the commitment step of glycolysis (as to not make excess ATP)
What factors that determine activity of enzymes?
Phosphorylation through a kinase
Dephosphorylation through phosphatase
Combining with a regulatory protein
Enzyme binding to a ligand (allosteric effector)
The concentration of substrate
Degrade protein into its amino acids (ubiquitin (marker); proteasome)
Sequester enzyme until it is needed
Regulating expression of gene by transcribing specific genes
Degradation of mRNA
Regulational translation
Regulation via extracellular signal
What is an example of the regulation of an alternative pathway?
Regulation of phosphofructokinase 1 and fructose 1,6-Bisphosphate
Go glycolysis if ADP and AMP is high, and if ATP and citrate is low
Go gluconeogenesis if AMP is low
What else can glycolysis be regulated by?
Insulin can change levels of proteins that change level of glycolysis
What is the first step of glycogen breakdown?
Remove one residue of glucose 1-phosphate, catalyzed by glycogen phosphorylase
Pi acts as a phosphate group on the one position
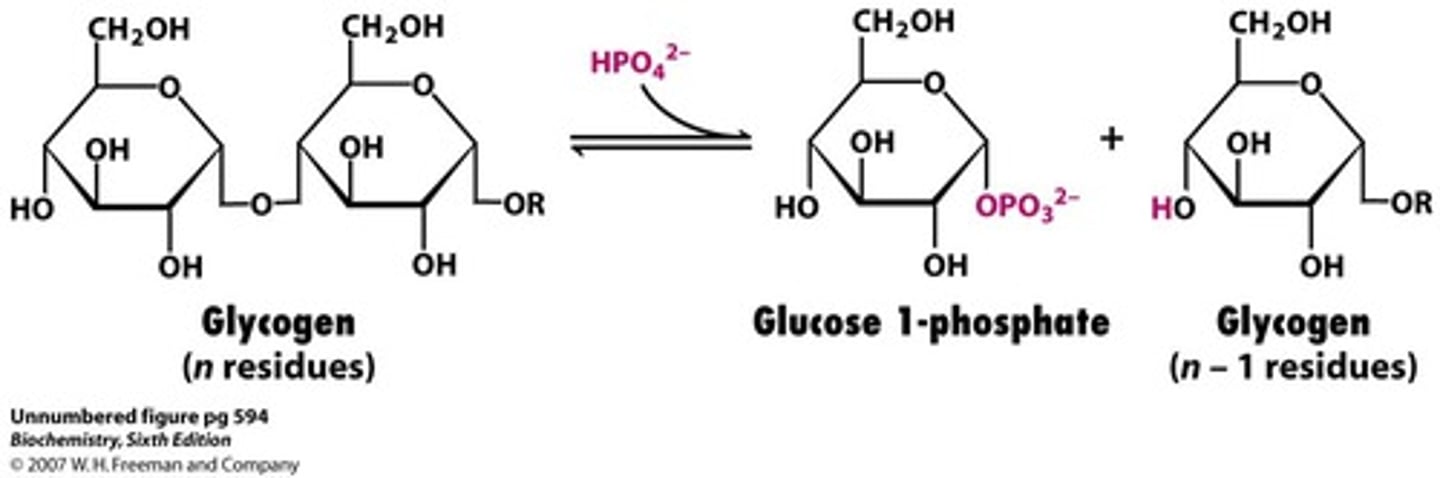
What is the second step of glycogen breakdown?
Once there are no more reducing ends, the transferase activity of the debranching enzyme transfers some residues to the larger chain
Glucosidase of debranching enzyme transfers glucose off branch
What is the third step of glycogen breakdown?
Phosphoglucomutase converts G1P to G6P
Donates phosphate group to G1P at the 6 position to turn it to Glucose 1,6-bisphosphate, and then removes the phosphate at the 1 position
How is glycogen synthesized?
Start with a sugar phosphate and NTP
Join sugar to the NTP to create pyrophosphate (PPi) and a sugar nucleotide (NDP-sugar) using NDP-sugar pyrophosphorylase
Next, PPi gets cleaved using inorganic pyrophosphatase and create 2 Pi
This sugar base (UDP-glucose) is used to combine with the nonreducing end of a glycogen chain that has n residues (when n>4), using the enzyme glycogen synthase and releasing a UDP in the process
Lastly, the glycogen-branching enzyme is used to add the branches to the glycogen molecule when the chain is long enough
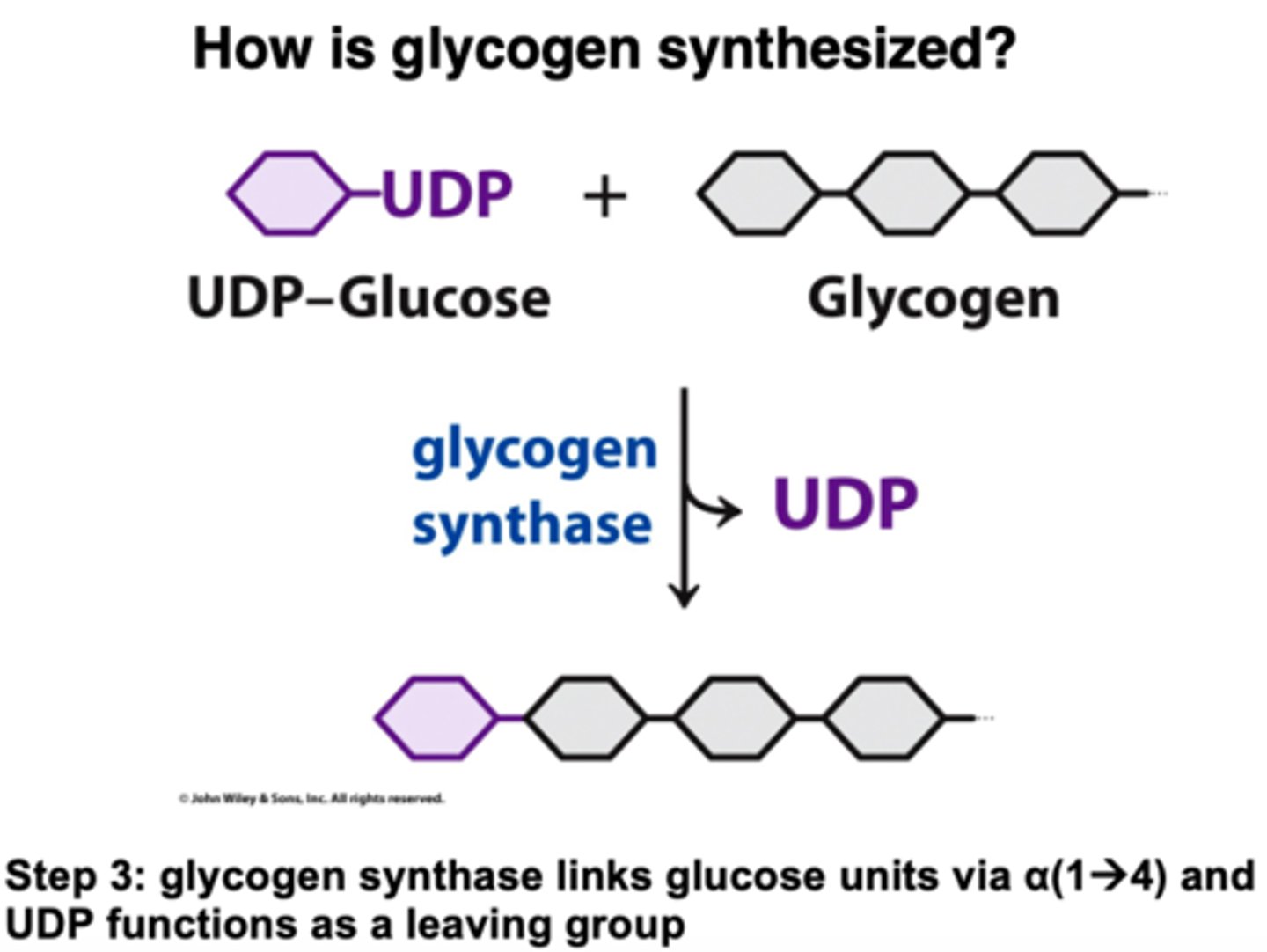
How is glycogen itself started?
Glycogen itself is started using the protein glycogenin
Catalyzes the synthesis of a short glycogen chain, about 8 glucose residues, that are covalently attached to the enzyme
From there, branches are added
What are the three major stages of cellular respiration?
Acetyl CoA production
Acetyl CoA oxidation
Electron transfer and oxidative phosphorylation
What is Acetyl CoA Production?
Breakdown of amino acids, fatty acids, and glucose (technically pyruvate because glycolysis) to create acetyl CoA
Pyruvate breaks down into Acetyl-Coa via pyruvate dehydrogenase complex, producing CO2 in the process
Generates some ATP, NADH, and FADH2
How is pyruvate converted to acetyl CoA?
pyruvate dehydrogenase complex
Requires five coenzymes
The net reaction is the oxidative decarboxylation of pyruvate
One carbon is released as carbon dioxide
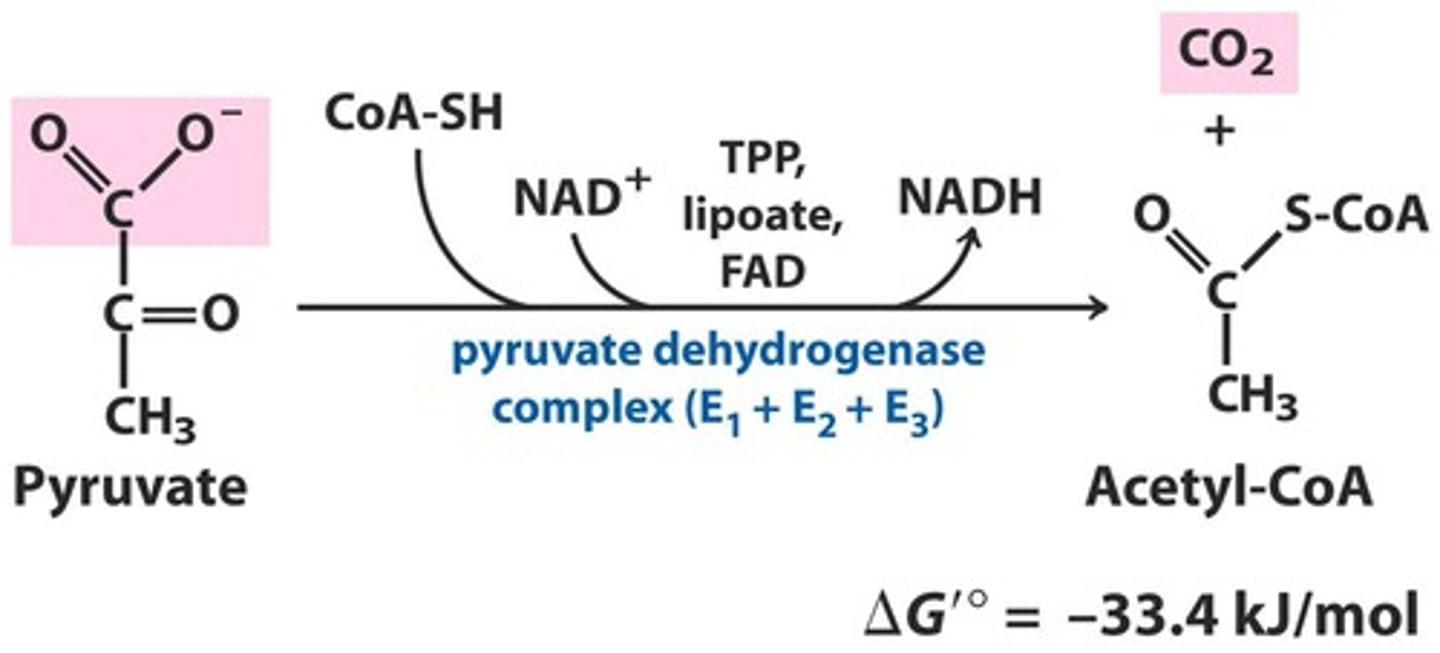
What is pyruvate dehydrogenase complex (PDC)?
Pyruvate dehydrogenase (E1)
Dihydrolipoyl transacetylase (E2)
Dihydrolipoyl dehydrogenase (E3)
How does PDC minimize side reactions?
Short distance between catalytic sites allows channeling of substrates from one site to another
How is PDC regulated?
Regulated by ATP
What are the five steps of oxidative decarboxylation of pyruvate?
Decarboxylation of pyruvate to an aldehyde
Oxidation of aldehyde to a carboxylic acid
Formation of acetyl CoA
Reoxidation of the lipoamide cofactor
Regeneration of the oxidized FAD cofactor
What are the co-substrates of the oxidative decarboxylation of pyruvate?
NAD+ and CoA-SH
What are the prosthetic groups of the oxidative decarboxylation of pyruvate?
TPP, lipoyllysine, and FAD
What is the function of CoA?
Accept and carry acetyl groups
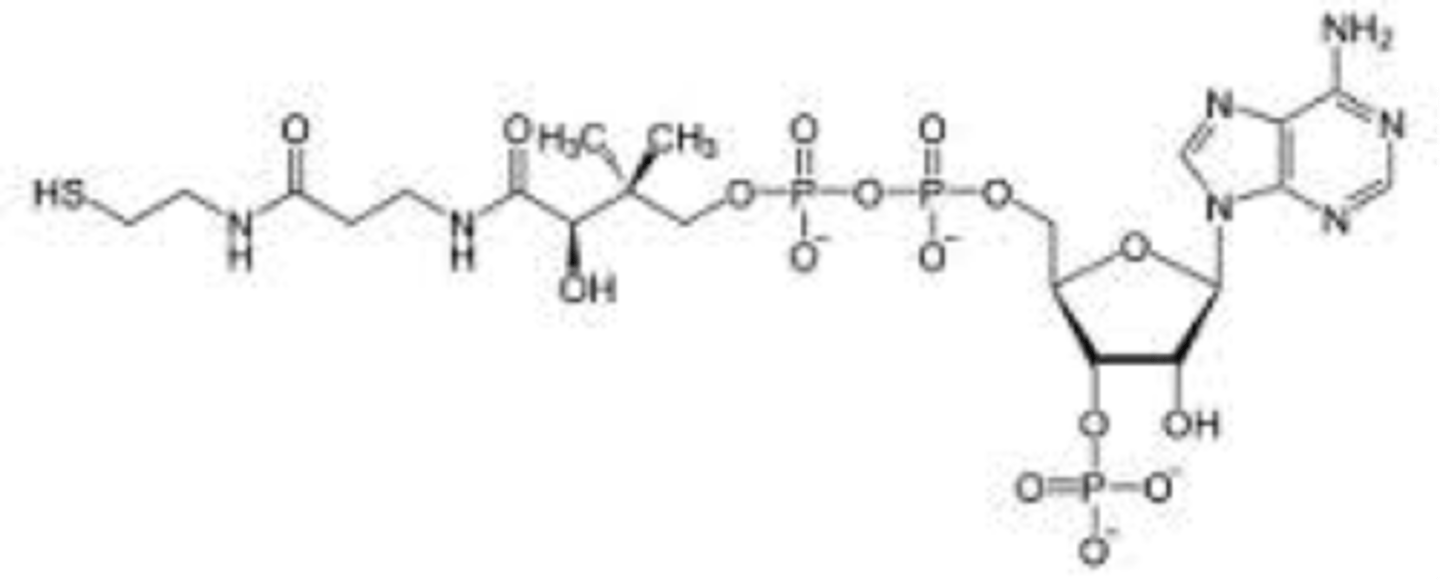
What is lipoyllysine?
Prosthetic group of oxidative decarboxylation of pyruvate
Helps reduce FAD
Lipoic acid is covalently linked to the enzyme via lysine
What is the first step of the citric acid cycle?
C-C bond formation to make citrate
Only step with C-C bond formation
Essentially irreversible
Combines Acetyl-Coa with Oxaloacetate, and uses citrate synthesis with water to release CoA-SH, producing citrate
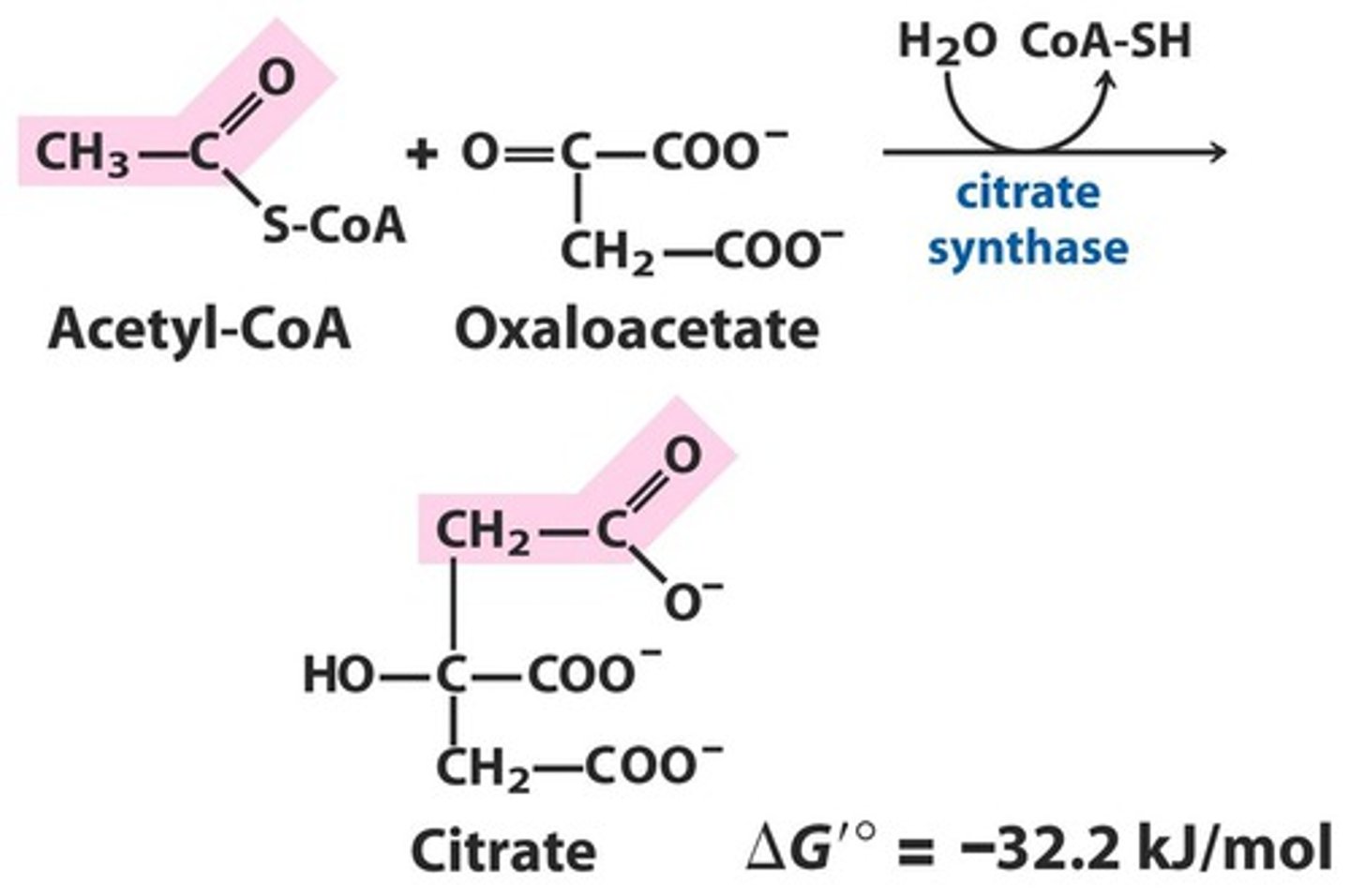
What is the relationship between citrate synthase and induced fit?
Conformational change upon binding oxaloacetate
Open conformation: Free enzyme does not have a binding site for acetyl CoA
Closed formation: Binding of oxaloacetate creates site for binding of acetyl CoA, meaning the reactive carbanion is protected in the closed conformation
This means that oxaloacetate binds first to the enzyme
What is the second step of the citric acid cycle?
Isomerization via dehydration, followed by hydration
Citrate is isomerized by aconitase
Citrate is a tertiary alcohol, making it a poor substrate for oxidation
Elimination of water from citrate gives a cis C=C bond
Addition of water to cis-aconitate is stereospecific
Isocitrate, a secondary alcohol, is a good substrate for oxidation
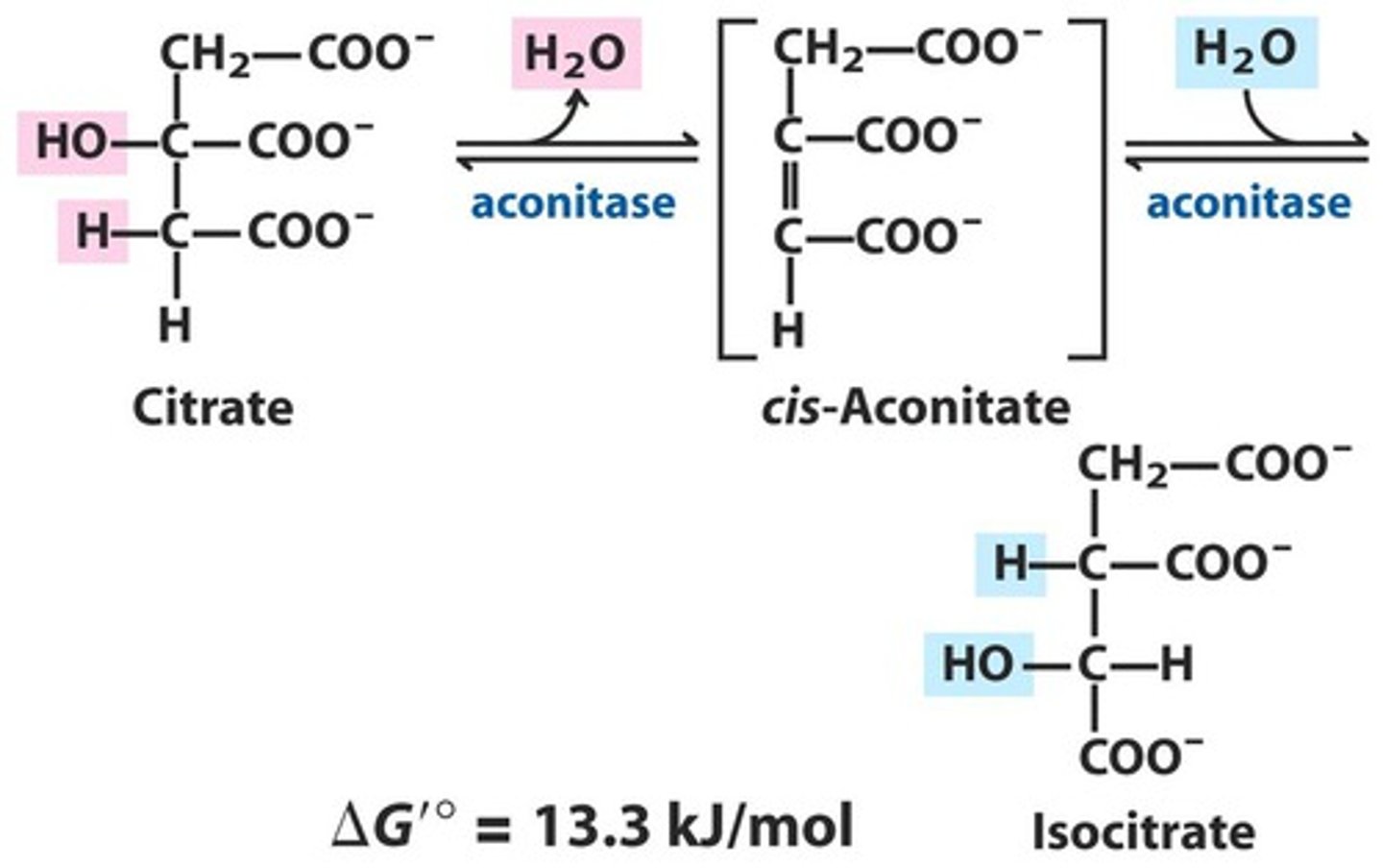
What lets aconitase remove water?
The iron-sulfur center removes water from the citrate and subsequent additions to cis-aconitate are catalyzed by the iron-sulfur center
What are steps three and four combined in the citric acid cycle?
Oxidative decarboxylation
Gives 2 NADH
What is the third step of the citric acid cycle?
Isocitrate dehydrogenase reaction
Oxidize alcohol to ketone by transferring hydride from the C-H of the alcohol to the nicotinamide cofactor
This is done by reducing NAD(P)+ to NADPH + H+, releasing CO2 in the process
Forms a-Ketoglutarate from isocitrate
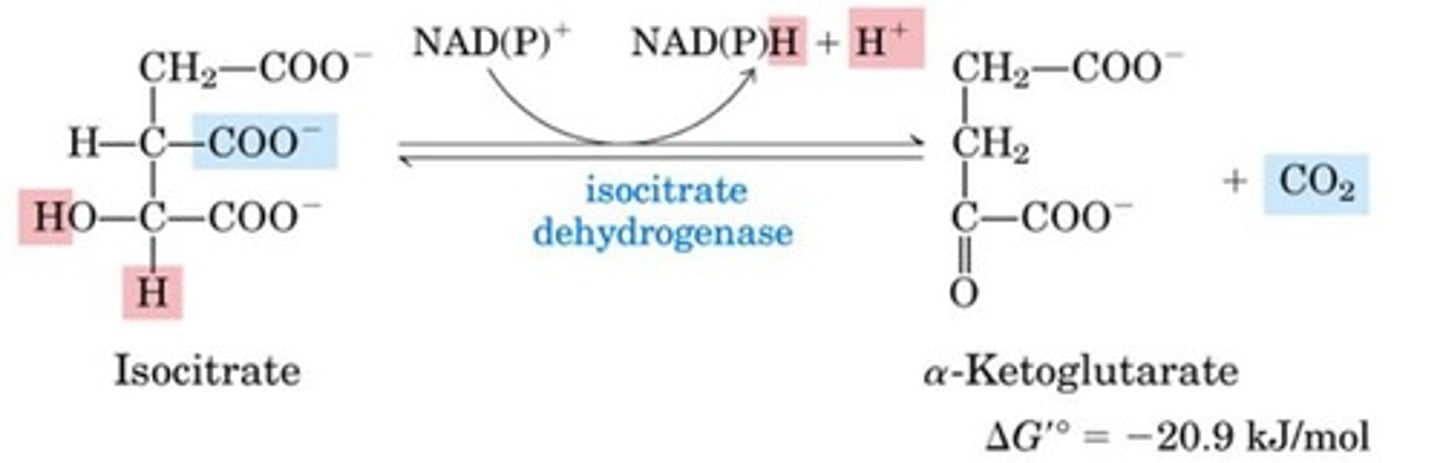
What is the fourth step of the citric acid cycle?
Oxidation of a-ketoglutarate
Enzyme: a-ketoglutarate dehydrogenase complex
This multiprotein complex resembles pyruvate dehydrogenase complex
Same coenzymes, and identical mechanisms
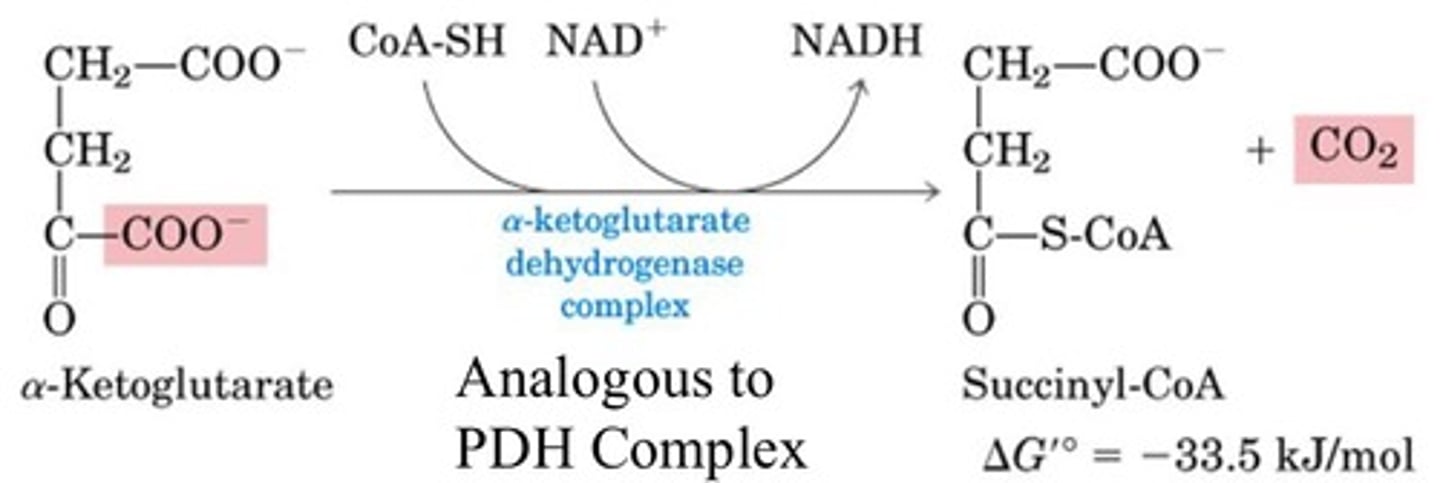
What is the fifth step of the citric acid cycle?
Substrate-level phosphorylation gives GTP
Succinyl-CoA to Succinate via succinyl-CoA synthetase
Produces GTP, which can be converted to ATP
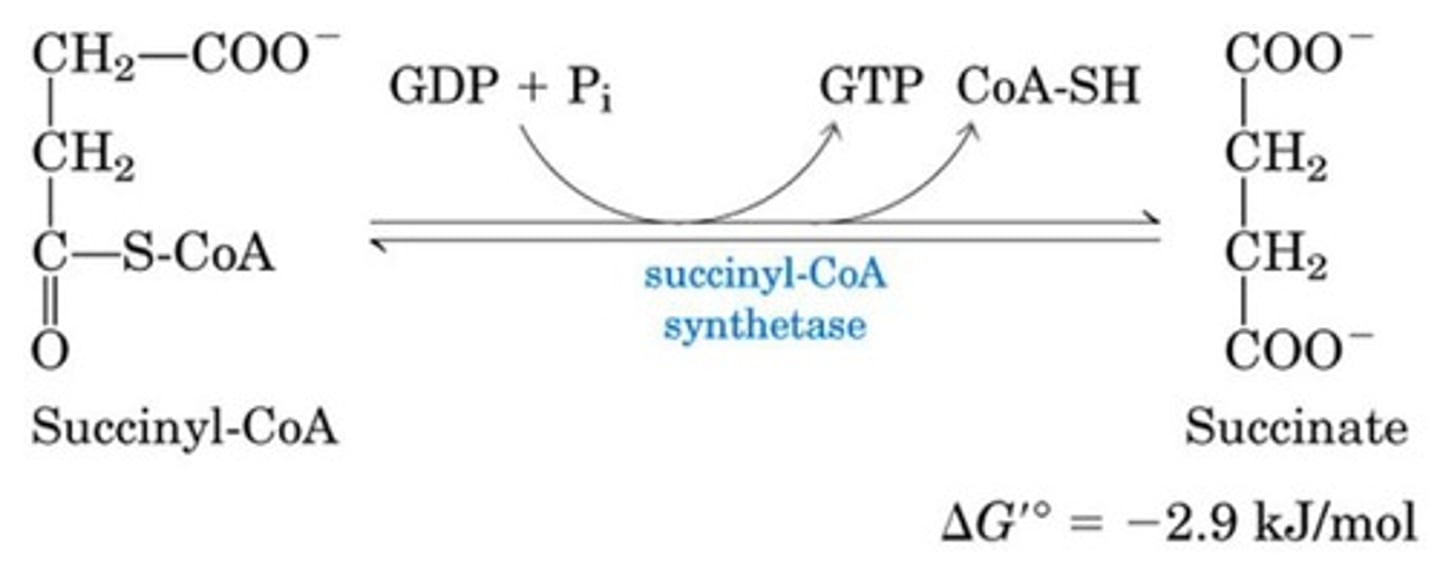
What is the sixth step of the citric acid cycle?
Dehydrogenation gives reduced FADH2
Catalyzed by the succinate dehydrogenase, which means that FAD acts a the redox cofactor and gets reduced to FADH2
FADH2 passes electrons to coenzyme Q, which the now reduced coenzyme QH2 is used to make ATP
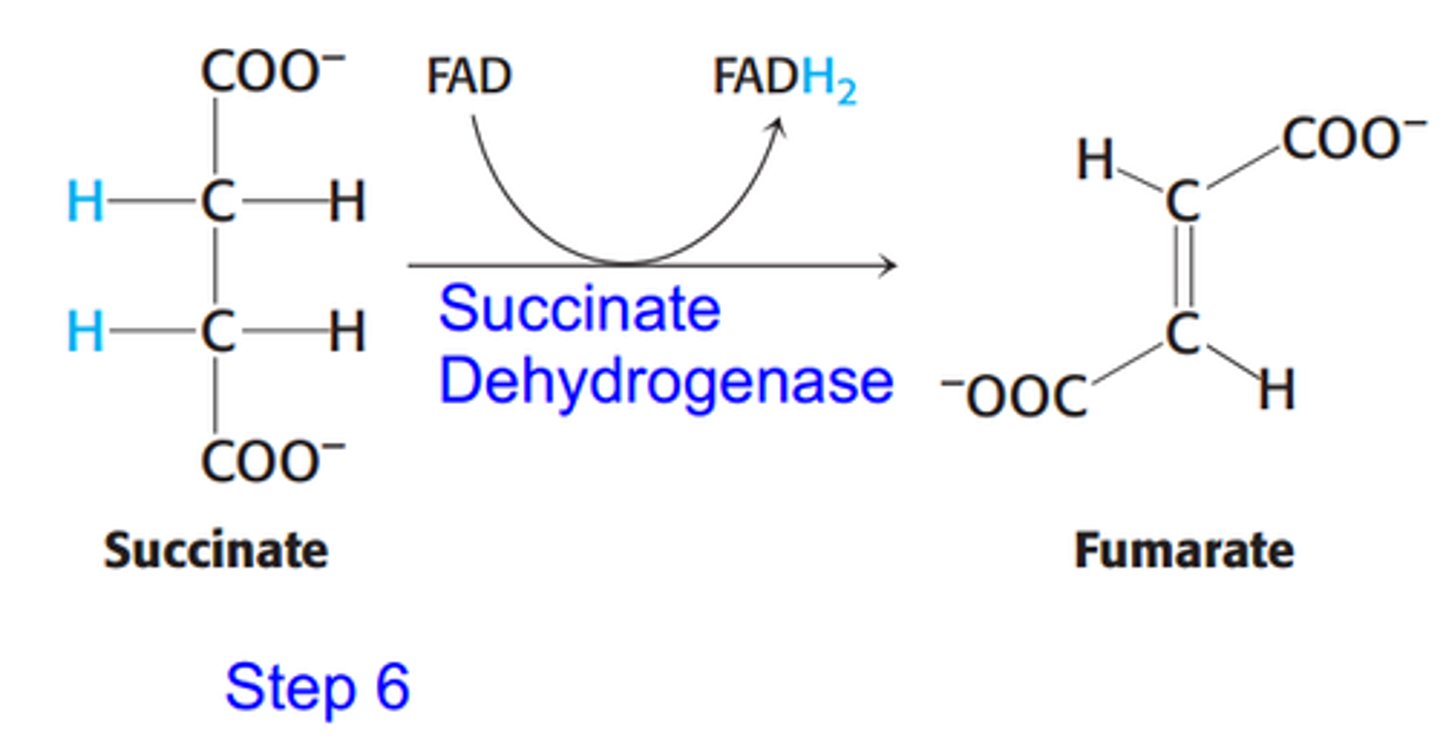
What is the seventh step of the citric acid cycle?
Hydration
Fumarate to Malate
Fumarase is highly stereospecific
OH- adds to fumarate, then H+ is added to the carbanion
Net effect: trans addition of water
This reaction is reversible!
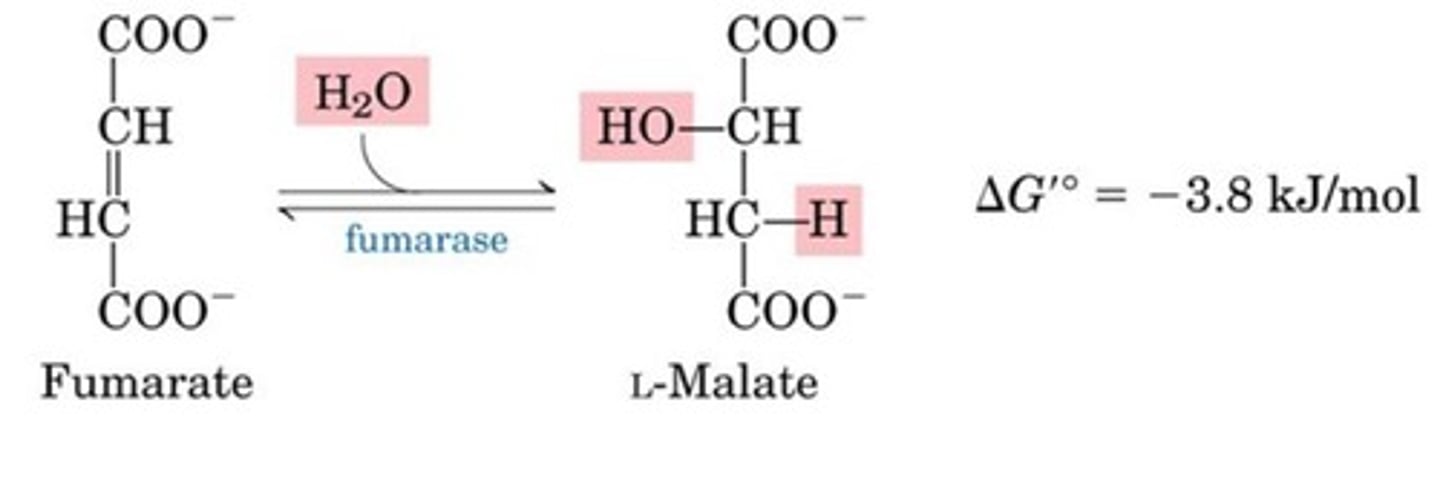
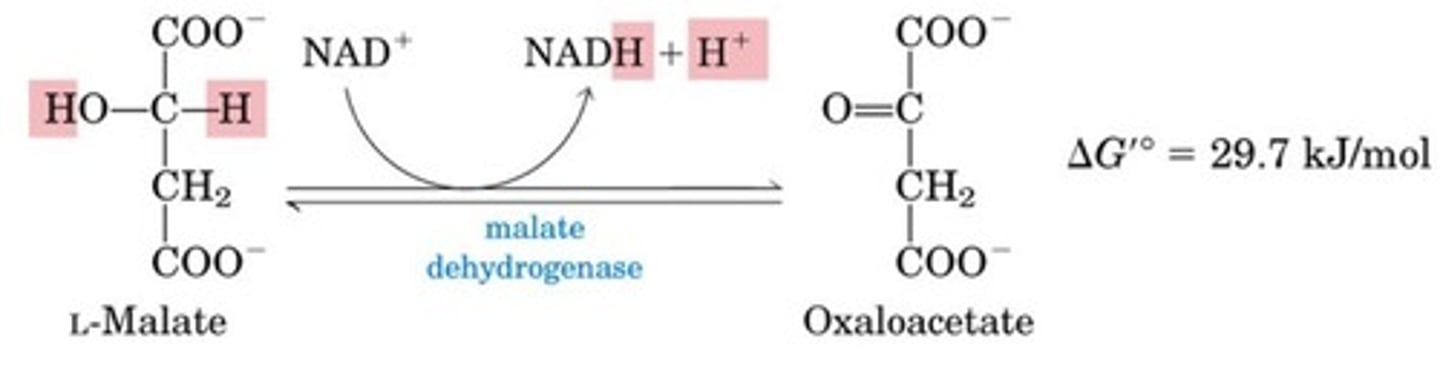
What is the eighth step of the citric acid cycle?
Dehydrogenation to give NADH
Oxidation of malate to oxaloacetate
NAD+ is the factor that is reduced to NADH + H+
This reaction is thermodynamically unfavorable reaction at standard conditions
This goes forward even though free standard energy change is positive, because oxaloacetate is very quickly used up
What are the products of the citric acid cycle?
Three NADH, one GTP (ATP), one FADH2, and two CO2
The oxaloacetate produced is reused in the first step
What are the net effects (not products) of the citric acid cycle?
Acetyl-CoA + 3NAD+ + FAD + GDP + Pi + 2H2O -> 2CO2 + 3NADH + FADH2 + GTP + CoA + 3H+
Carbons of acetyl groups in acetyl-CoA are oxidized to CO2
Electrons from this process reduce NAD and FAD
One GTP is formed per cycle, which can be later converted to ATP
Intermediates in the cycle are not depleted
How is the breakdown of pyruvate to acetyl-CoA regulated?
ATP, acetyl-CoA, NADH, and fatty acids decrease activity
AMP, CoA, NAD+, and Ca2+ increase activity
How is the conversation of Acetyl-CoA to citrate regulated?
NADH, succinyl-CoA, citrate, and ATP decrease activity
ADP increase activity
How is the conversation of isocitrate to a-Ketoglutarate regulated?
ATP decreases activity
Ca2+, ADP increases activity
How is the conversation of a-Ketoglutarate to Succinyl-CoA regulated?
Succinyl-CoA, and NADH decrease activity
Ca2+ increases activity
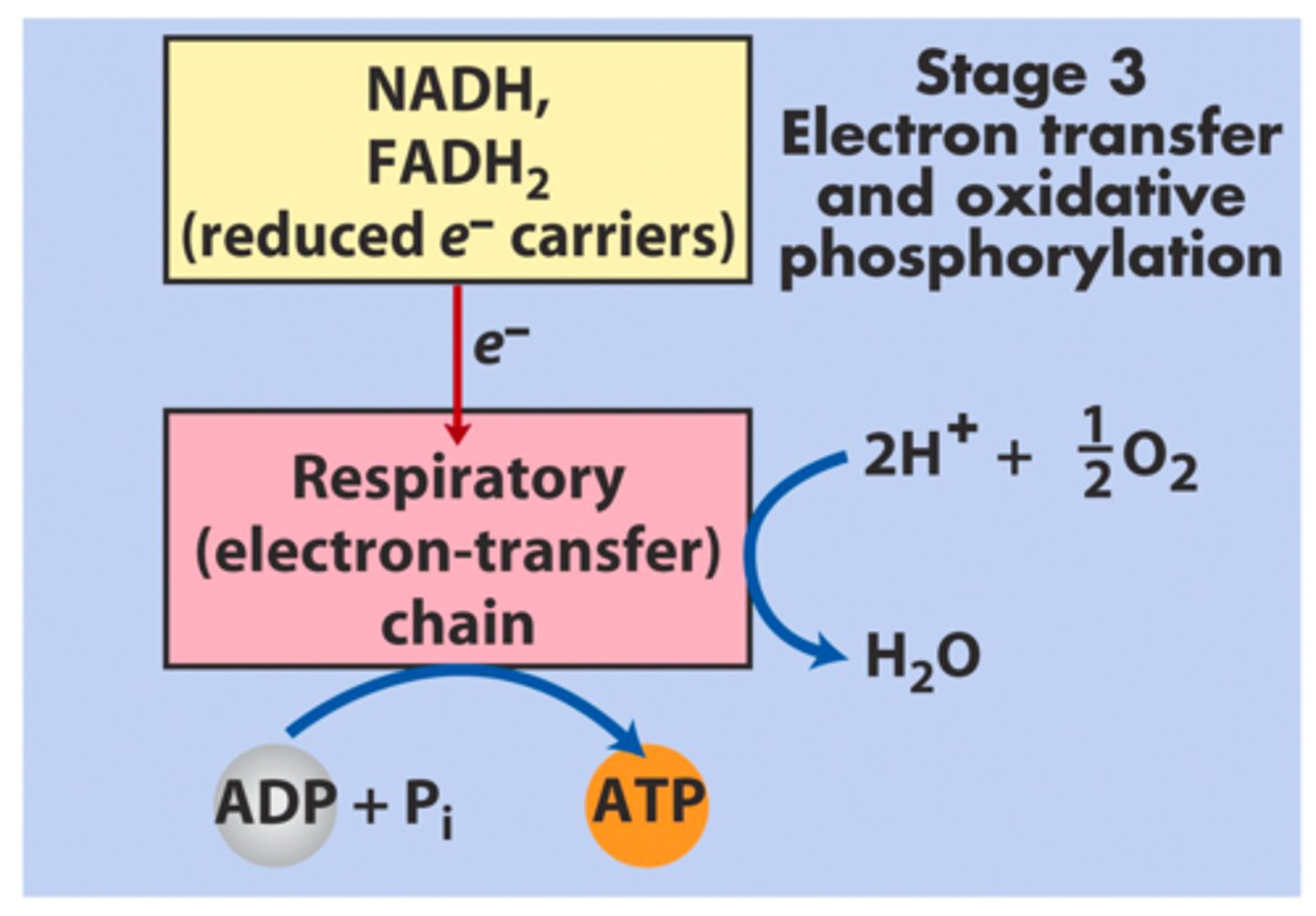
What is the end product of electron transfer and oxidative phosphorylation?
Lots of ATP
How does electron transfer and oxidative phosphorylation occur?
Uses reduced electron carriers (NADH, FADH2) and 2H+ and 1/2 O2 to go through the electron transport chain and combine ADP with Pi
Where does the citric acid cycle occur in eukaryotes?
Glycolysis occurs in the cytoplasm
The citric acid cycle occurs in the mitochondrial matrix
The except is succinate dehydrogenase, which is in the the inner membrane
Oxidative phosphorylation occurs in the inner membrane
What is the total amount of ATP produced from breaking down 1 glucose?
30-32 ATP molecules, from glycolysis, the pyruvate dehydrogenase complex reaction, citric acid cycle, and oxidative phosphorylation
Glyceraldehyde 3-phosphate dehydrogenase
Enzyme that produces NADH during glycolysis.
Phosphoglycerate kinase
Catalyzes substrate-level phosphorylation in glycolysis.
Phosphohexose isomerase
Isomerase that converts glucose-6-phosphate to fructose-6-phosphate.
Phosphofructokinase-1
Enzyme that catalyzes ATP investment in glycolysis.
Aldolase
Enzyme that splits hexose into two three-carbon compounds.
Triosephosphate isomerase
Isomerase that interconverts dihydroxyacetone phosphate and glyceraldehyde 3-phosphate.
Pyruvate kinase
Catalyzes ATP production in glycolysis.
Phosphoglycerate mutase
Enzyme that rearranges 3-phosphoglycerate to 2-phosphoglycerate.
Enolase
Catalyzes the conversion of 2-phosphoglycerate to phosphoenolpyruvate.
Citrate
Starting molecule in aconitase reaction before conversion to isocitrate.