lecture 21: drug solubility and dissolution rate 4
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
41 Terms
what are surfactants?
components that can decrease surface tension allowing miscibility of 2 phases which are otherwise unmiscible
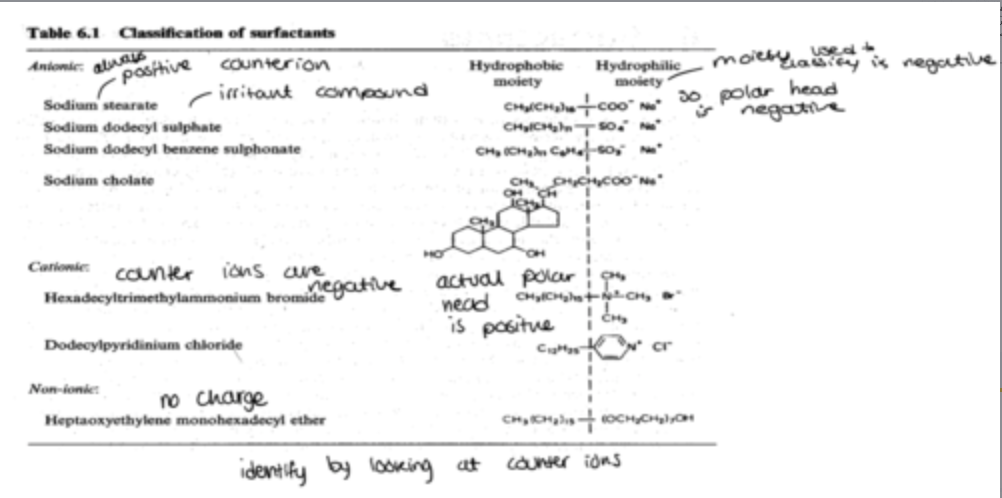
what are the classifications of polar surfactants?
anionic
cationic
non-ionic
how can the classification of surfactants be identified?
cationic: counter ions are negative
anionic: counter ions are positive
what are zwitterions?
molecules that contain both positive and negative charges on the
mostly amino acids
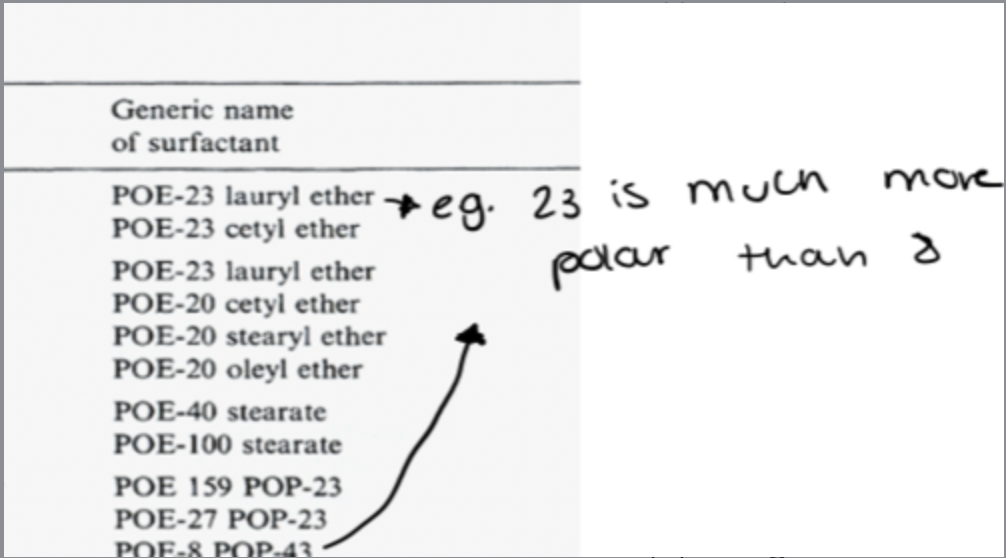
what are the features of non-ionic surfactants?
no cationic or anionic polar heads so we need to create polarity by using polyoxyethylene chains
they accumulate on the head making it bigger
the more polyoxyethylene chains, the more polar the compound as it can help pull big aliphatic chains into the water
the number of groups = POE number
what are the applications of anionic surfactants?
widely used bcs they are cheap
toxicity: so only used for external applications
O/W emulsifiers, they decrease interfacial tension between oil and water
what are the applications of cationic surfactants?
disinfectant, preservative properties
O/W emulsifiers
toxicity
what are the applications of non-ionic surfactants?
best option
O/W and W/O emulsifiers
low toxicity and low irritancy
oral and parenteral use
how do ionic surfactants affect parenteral use?
can cause haemolysis of RBC and destruction of T lymphocyte cells
how do non-ionic surfactants affect parenteral use?
consider phospholipids, if they can pass through or not
consider polysorbates(common emulsifiers)
cremaphor EL has been shown to cause anaphylactic shock therefore restricted use
toxicity related to residual contamination of ethylene oxide
the more residual contamination the more observed toxicity.
what are examples of surface active drugs?
- hydrophobic portion:
aromatic of heterocyclic ring system
- tranquilizers:
chlorpromazine
- antidepressants:
imipramine
- antihistamine:
diphenydramine
-antibiotic:
penacillin G
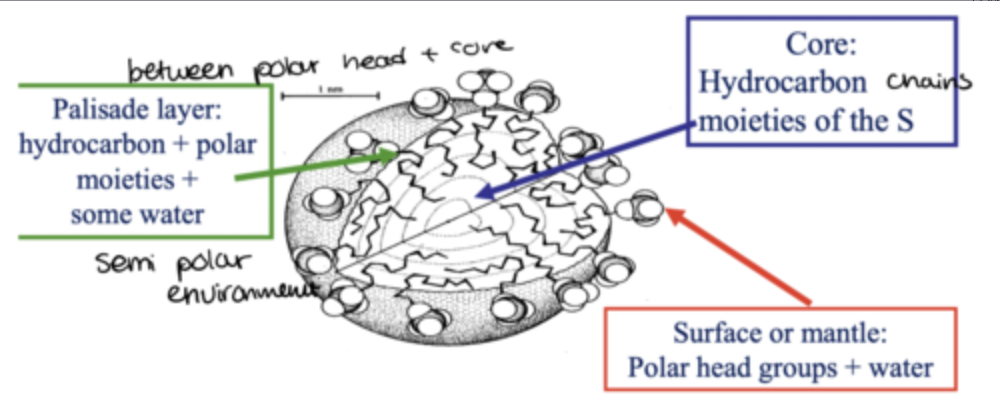
what happens as the quantity of surfactants increases in water?
good quantity of surfactants in water, no more adsorbtion occurs, leads to aggregation at a specific concentration CMC
monolayers of surfactants are formed
in such a system, if you add drugs they can also be solubilised when micelles form
these drugs can be of different nature: polar, non-polar, semi-polar
depending on their nature, they associate with different parts of the micelles when solubilised
diagram of micelle:
how do polar drugs associate with micelles?
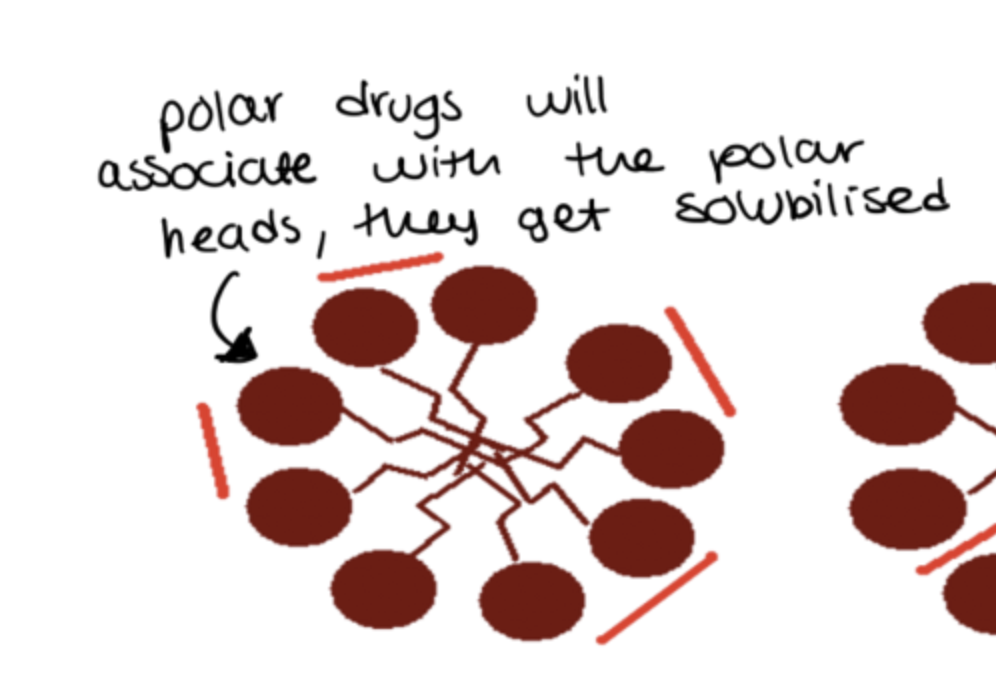
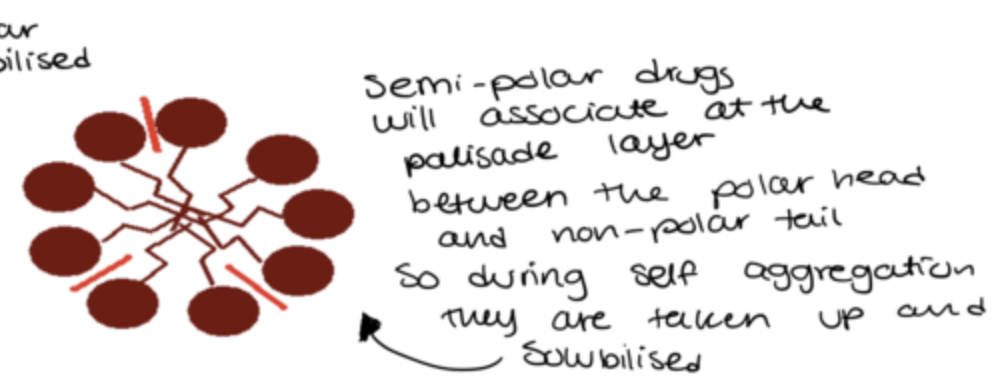
how do semi-polar drugs associate with micelles?
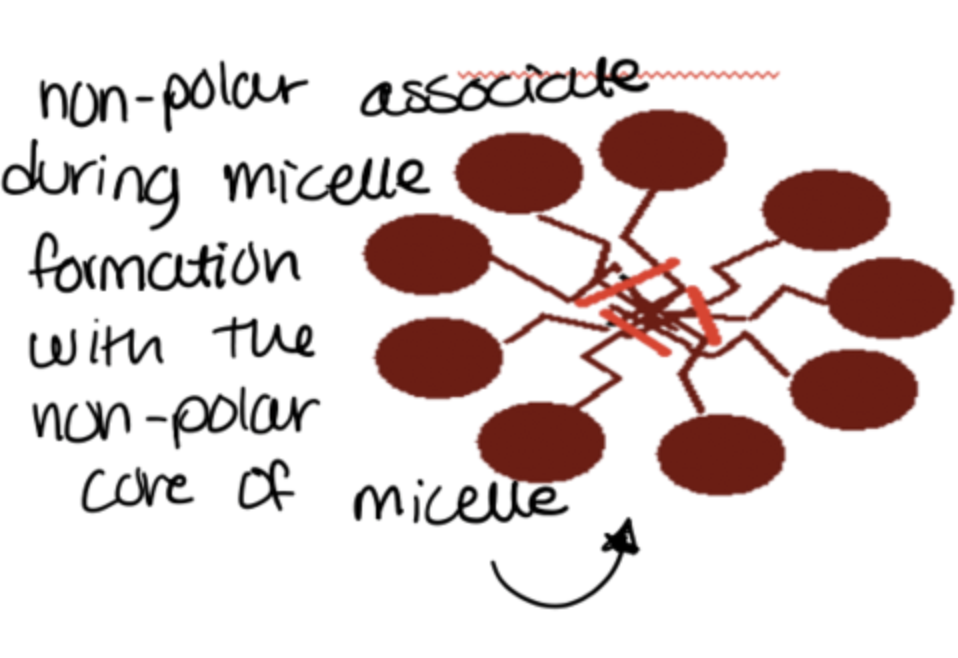
how do non-polar drugs associate with micelles?
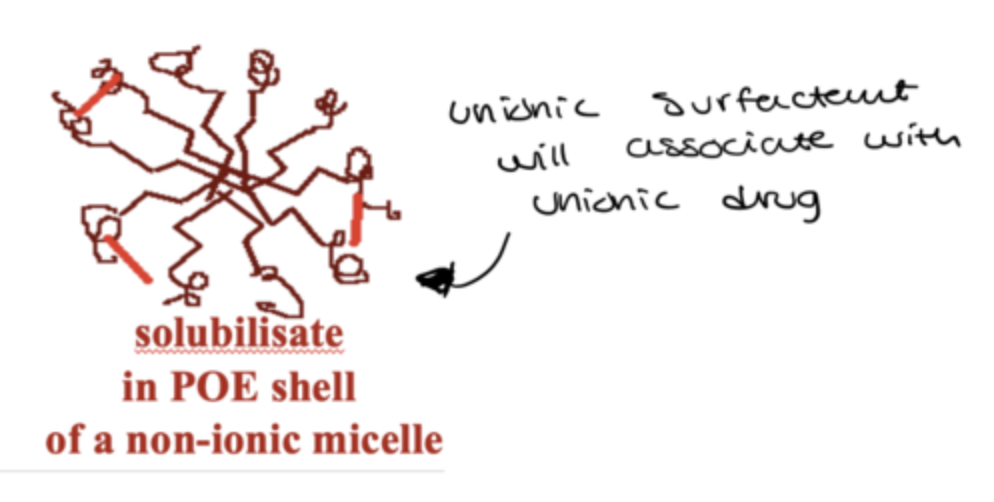
what happens when an unionic surfactant associates with an unionic drug?
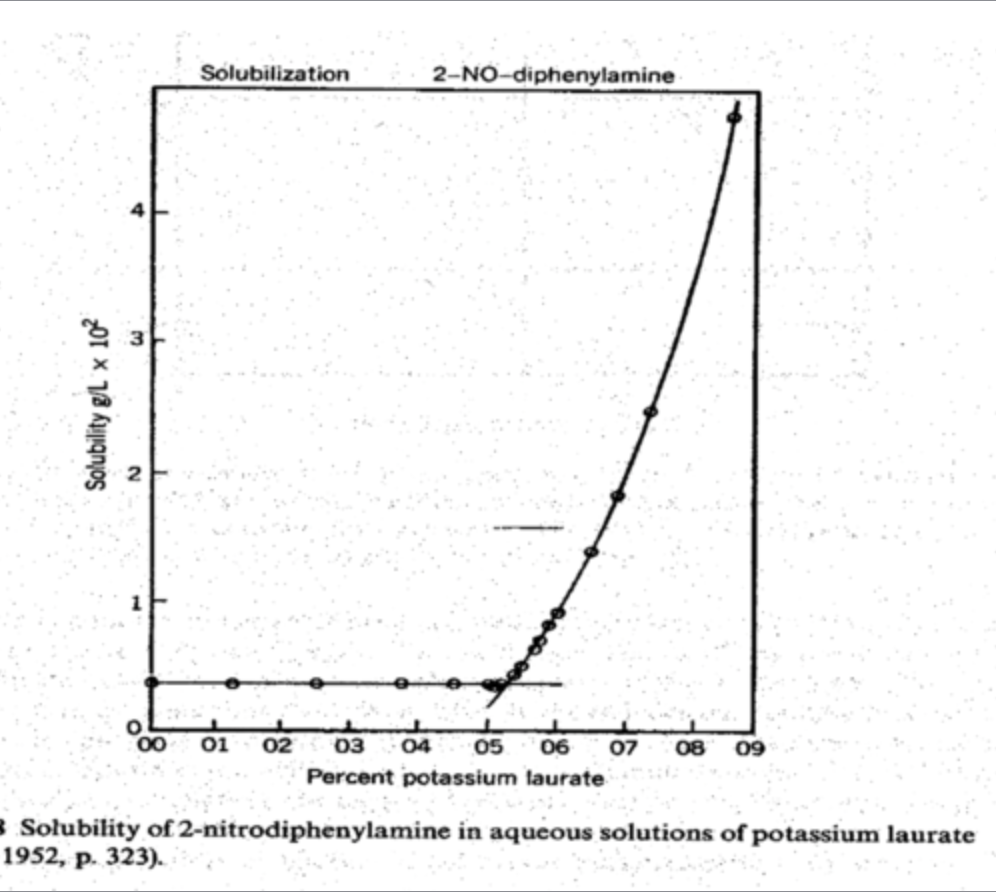
example of CMC graph:
how can you pick which surfactant to use?
you must calculate the solubilisation capacity and compare them.
Sm= molar solubility of the solute in the micelle
Cmic= molar concentration of micellar surfactants
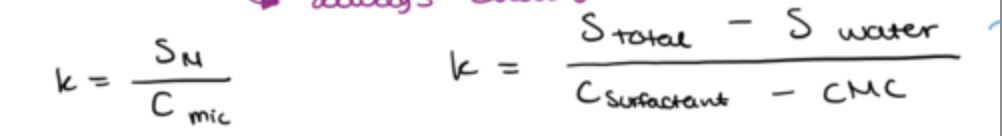
what is molar solubilisation capacity(k)?
the number of moles of a solute that can be solubilised by 1 mole of micellar surfactant
how do u calculate the total solubility?
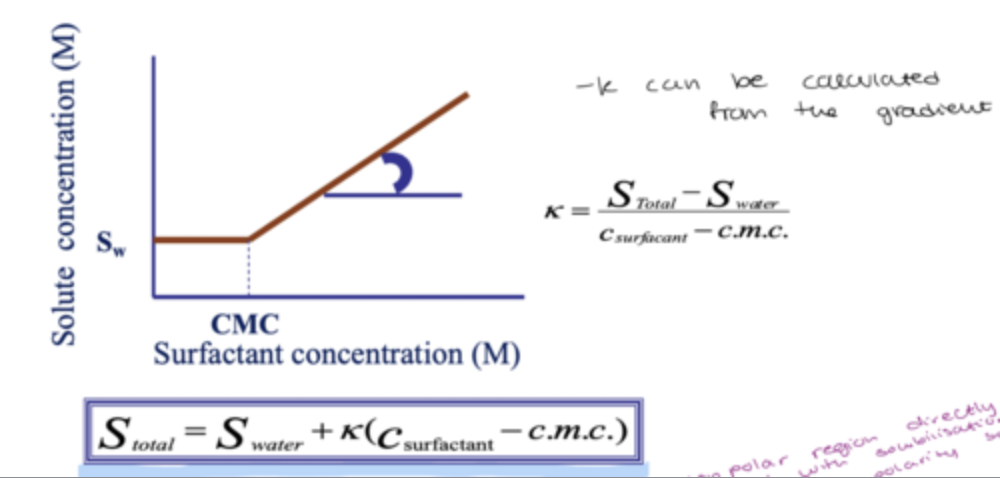
how can the surfactant structure affect the solubility?
non polar region is directly related with solubilisation capacity of low polarity solutes
1. increasing HC chain:
larger nonpolar region: solubilise more of non-polar solute
large reservoir in the micelle core, larger core, higher capacity to solubilise therefore decreases CMC
2. introduction of polar groups, a double bond
equivalent to decrease length of chain therefore having polar groups in aliphatic chains is not a good thing
3. branched surfactants give smaller micelles
semi polar:

how are surfactants selected?
amount of surfactant that can be placed in water. very long chains are not effective surfactants due to low solubility
ability to solubilise a solute. very short chain therefore has a high CMC
because you need a high conc of surfactants to make the system unstable so you can get micelle formation
both long and short are inconvenient therefore we want equilibrium
in practice a balance is required
12 to 16 carbons or 18 with a double bond
this provides a low CMC and sufficient water solubility
high chain length= reduced CMC and reduced solubility
low CMC corresponds to low surfactant solubility which reduces the amount of surfactant that can be used
increasing chain length in 2 carbons decreases solubility by 10 fold
what are features of surfactants with long HC chains?
they are not soluble but can solubilise a lot of non-polar drugs
the lipophilic part limits the solubility in water
the hydrophile must provide enough interaction with water to bring the insoluble lipophile into solution - A - or + ion can bring a 16-C chain into water at room temperature
what happens as a chain length increases?
solubility decreases
surface activity becomes more pronounced
longer the HC chain, greater tendency of the surfactant molecules to adsorb at the surface and lower the surface tension
What is Lundelius' rule?
any factor that tends to decrease solubility of the surfactant promotes surface activity
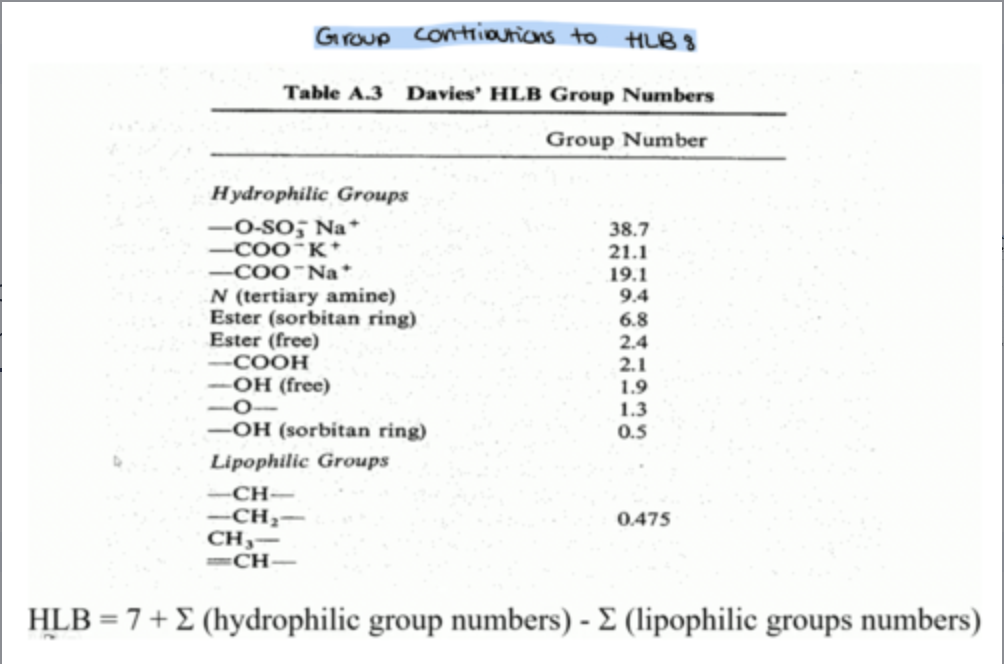
how do you calculate the HLB scale for a mixture?
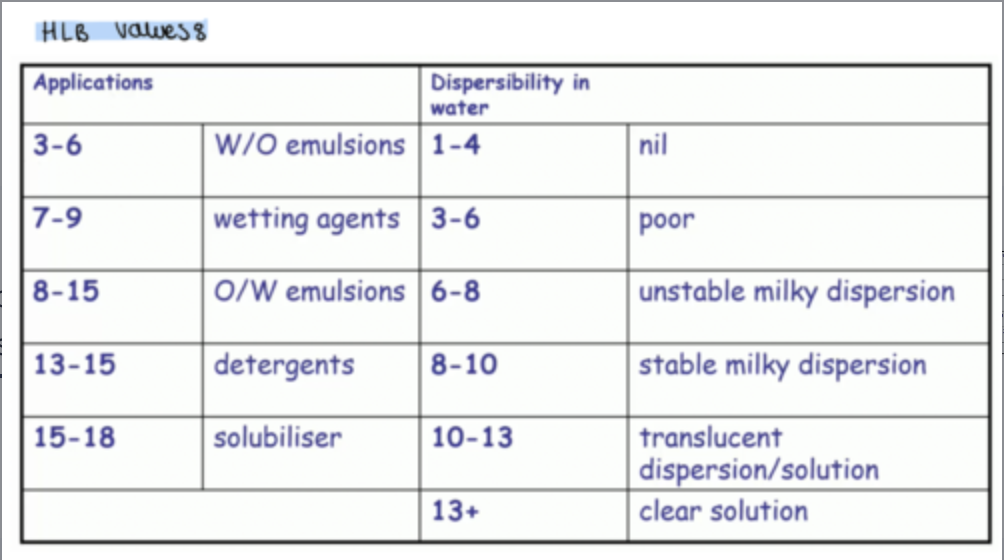
high HLB numbers indicate a surfactant exhibiting mainly polar or hydrophilic properties
low HLB numbers represent lipophilic or non-polar properties

How do you calculate HLB for mixtures(formula)?
what can hydrophilic water soluble surfactants be used for?
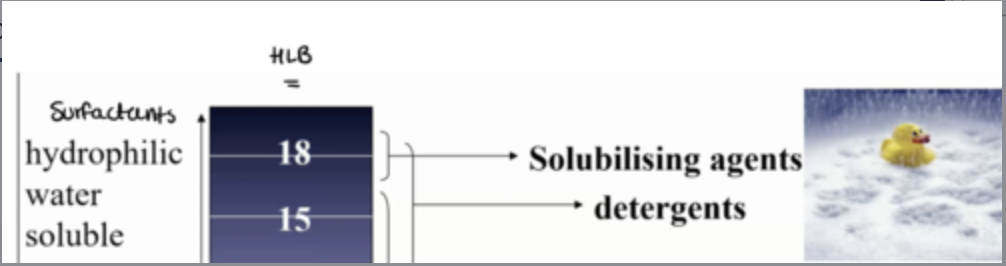
how can water dispersible surfactants be used?
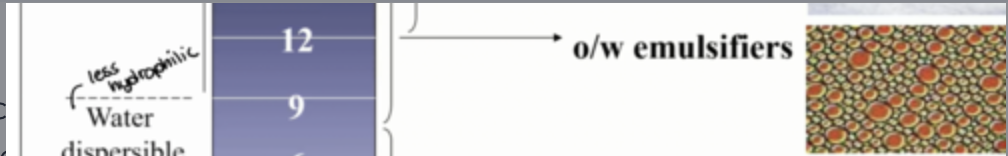
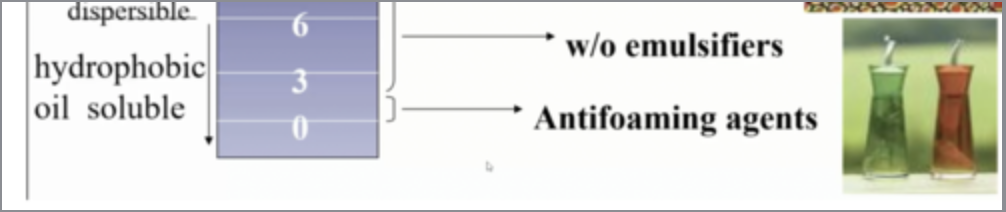
how can hydrophobic oil soluble surfactants be used?
what do the different HLB values correspond to?
how can the HLB of a mixture of surfactants be measured?

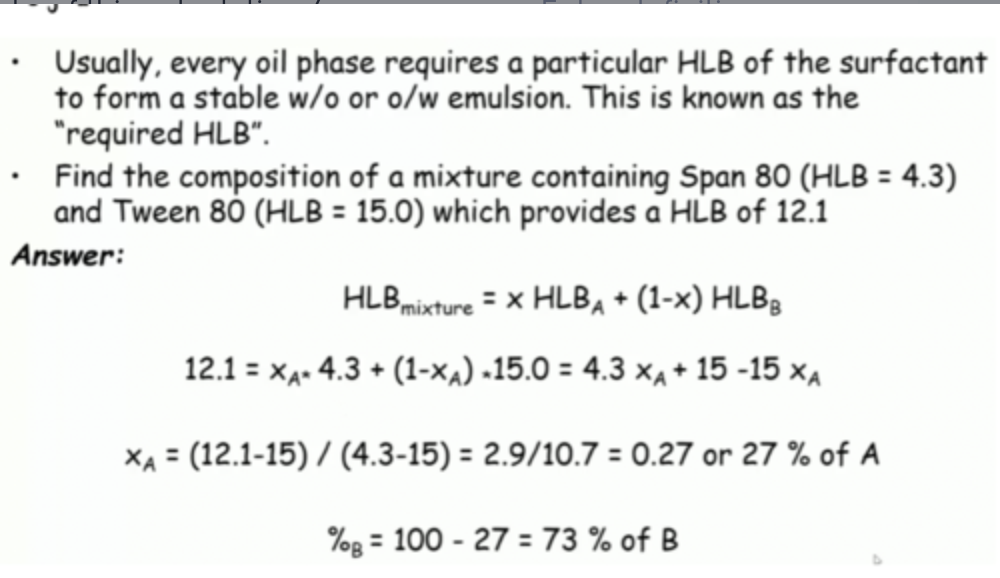
give an example of this calculation:

how are surfactants used to stabilise emulsions?
using a surfactant reduces the interfacial tension between oil and water so they are used to stabilise emulsions(emulgent)
each type of oil requires an emulgent of a particular HLB number in order to ensure a stable emulsion
how to calculate HLB required?
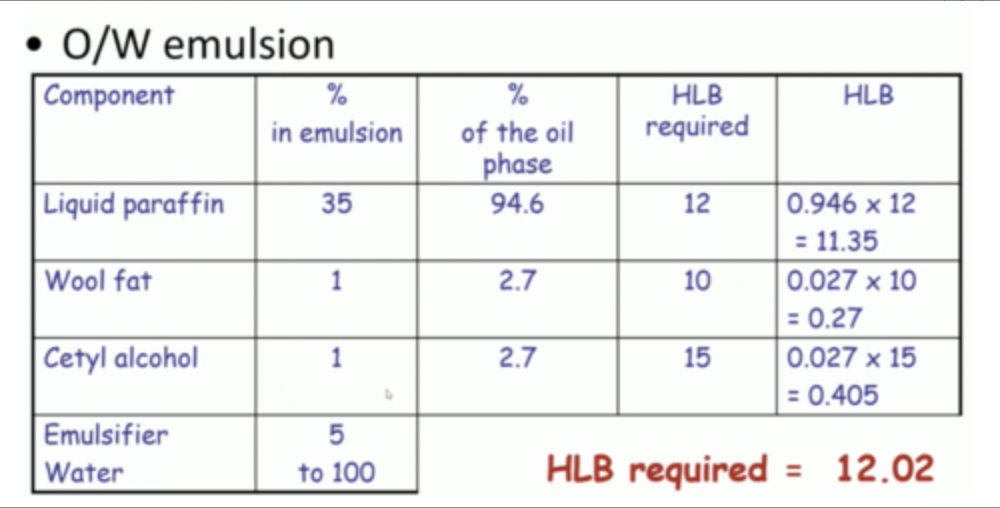
example of this calculation:4
what is phase inversion temperature(PIT)?
the temperature in which some of the surfactants change from o/w emulsifier to w/o emulsifier as they become less soluble in water
at high temps, hydrogen bonds are weakened by thermal forces and the emulsifiers are less soluble in water
at the PIT, the hydrophilic and lipophilic nature just balance
why does the HLB of an emulsifier vary with temperature?
because the relative solubilities of the lipophile and hydrophile vary with temperature
effects are more pronounced for non-ionic surfactants as their solubility in water depends on hydrogen bonding
common non-ionic emulsifiers:
water soluble at low T, stabilise o/w emulsions
oil soluble at high T, stabilise w/o emulsions