Chemistry I Lesson 8: Salts and Buffers
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
CRB Knowing that Ka = [H3O+][A-]/[HA], write out the formula for the base dissociation constant Kb.
Kb = [B+][OH-]/[BOH]
Struggling to keep your MCAT equations straight? Simply conquer the 100 most important equations using Andrew's 100 Most Essential Equations Mastery Course @ https://mcatselfprep.com/course/andrews-equation-mastery-course/
What equation illustrates the relationship between the Ka of an acid and the Kb of its conjugate base?
Kw = Ka ⋅ Kb
Struggling to keep your MCAT equations straight? Simply conquer the 100 most important equations using Andrew's 100 Most Essential Equations Mastery Course @ https://mcatselfprep.com/course/andrews-equation-mastery-course/
What is the Kb of Cl- if the pKa of HCl is -7?
(A) 1 ⋅ 10^-21
(B) 1 ⋅ 10^-14
(C) 1 ⋅ 10^-7
(D) 1 ⋅ 10^7
(A) 1 ⋅ 10^-21
pKw = pKa + pKb
14 = -7 + pKb
21 = pKb
pKb = -log(Kb)
21 = -log(Kb)
Kb = 1 ⋅ 10^-21
Need help with MCAT math? Become an MCAT math wizard using Andrew's High-speed Math Mastery Course @ https://mcatselfprep.com/course/andrews-high-speed-math-mastery-course/
CRB In the hydrolysis of salts, a cation and anion will be freed and may interact with water. Which of the following cations would be a stronger acid than water?
(A) Na+
(B) NH4+
(C) Mg2+
(D) K+
(B) NH4+
Group 1 cations and larger group 2 cations do not react with water. Ammonium, on the other hand, will react with water and donate a proton.
CRB In the hydrolysis of salts, a cation and anion will be freed and may interact with water. Which of the following anions would be a stronger base than water?
(A) CN-
(B) Br-
(C) CH3CO2-
(D) NO3-
(A) CN-
The conjugate base to a strong acid will not react with water; the weaker that the acid is, the stronger its conjugate base is.
Both CH3CO2- and CN- are conjugate bases to weak acids (acetic acid and hydrogen cyanide, respectively). In this case, we must compare the acids' pKa's to water's pKa: acetic acid has a pKa of 4.75, whereas hydrogen cyanide has a pKa of 9.2.
Water has a pKa of 7, so only CN- is a stronger base than water.
CRB Which of the following salts will produce a basic solution when added to pure water?
(A) KCl
(B) NaClO
(C) HBr
(D) Na2SO4
(B) NaClO
Sodium is a group 1 cation, so it will not react with water. ClO-, however, is the conjugate base to a weak acid and will react with water.
CRB Which of the following very polar compounds will produce an acidic solution when added to pure water?
(A) KCl
(B) NaClO
(C) HBr
(D) Na2SO4
(C) HBr
You should recognize that HBr is a strong acid!

CRB Which of the following salts will NOT react with the water after hydrolysis?
(A) MgCl2
(B) Fe(NO3)3
(C) CuCl2
(D) NaNH2
(A) MgCl2
Copper (II) and Iron (III) are both stronger acids than water and will react with the water, and NH2- is a stronger base than water. On the other hand, Magnesium is a larger group 2 element and Cl- is the conjugate base to a strong acid, so neither the cation nor anion will react with the water!
You add CH3COO- to water. What do you predict will be the resulting pH? Why?
It will be greater than 7, because CH3COO- is the conjugate BASE of a weak acid.
If the Ka of CH3COOH is 1.8 ⋅ 10^-5, what is the pH of a .54 M solution of CH3COO-?
(A) 10.35
(B) 9.24
(C) 8.43
(D) 7.21
(B) 9.24
Ka⋅Kb = Kw
(1.8 ⋅ 10^-5)Kb = 1 ⋅ 10^-14
Kb = (1 ⋅ 10^-14)/(1.8 ⋅ 10^-5)
Kb = approx. 5 ⋅ 10^-10 (actual: 5.6 ⋅ 10^-10)
Kb = ([CH3COOH][OH-])/[CH3COO-]
5 ⋅ 10^-10 = (x^2)/(.54-x) but we can just assume that x is negligible compared to .54:
5 ⋅ 10^-10 = (x^2)/(.54)
approx 2.5 ⋅ 10^-10 = x^2
x = [OH-] = approx. 1.35 ⋅ 10^-5 (actual: 1.74 ⋅ 10^-5)
pOH = -log([OH-])
pOH = -log(1.35 ⋅ 10^-5)
pOH = approx. 4.65 (actual: 4.76)
pKw = pOH + pH
14 = 4.65 + pH
pH = approx. 9.35 (actual: 9.24)
Need help with MCAT math? Become an MCAT math wizard using Andrew's High-speed Math Mastery Course @ https://mcatselfprep.com/course/andrews-high-speed-math-mastery-course/
You add sodium acetate to a solution of acetic acid. What will happen to the pH and why?
(A) The pH will increase because an increased concentration of sodium ions will push the acid dissociation reaction backwards.
(B) The pH will increase because an increased concentration of acetate ions will push the acid dissociation reaction backwards.
(C) The pH will decrease because an increased concentration of sodium ions will push the acid dissociation reaction backwards.
(D) The pH will decrease because an increased concentration of acetate ions will push the acid dissociation reaction backwards.
(B) The pH will increase because an increased concentration of acetate ions will push the acid dissociation reaction backwards.
This is because the introduction of one of the products is shifting the equilibrium back towards reactants, according to Le Chatelier's principle.
True or false? Sodium acetate increasing the pH in a solution of acetic acid is an example of the common ion effect.
True. Sodium acetate increasing the pH in a solution of acetic acid is an example of the common ion effect.
How does the common ion effect relate to Le Chatelier's principle?
The common ion effect will shift the equilibrium by increasing the concentration of one of the products. This is an example Le Chatelier's principle, where the equilibrium adjusts to relieve the added stress of increasing the products' concentration.

What is the pH of a solution of 5.6 ⋅ 10^-1 M acetic acid (Ka = 1.8 ⋅ 10^-5) and 5.3 ⋅ 10^-2 M sodium acetate?
(A) 2.31
(B) 3.72
(C) 4.56
(D) 5.89
(B) 3.72
Ka = ([CH3COO-][H3O+])/[CH3COOH]
1.8 ⋅ 10^-5 = ([5.3 ⋅ 10^-2 + x][x])/[5.6 ⋅ 10^-1 - x] but x can be assumed to be negligible compared to our concentrations:
1.8 ⋅ 10^-5 = ([5.3 ⋅ 10^-2][x])/[5.6 ⋅ 10^-1]
1.8 ⋅ 10^-5 = approx. (1 ⋅ 10^-1)x
(1.8 ⋅ 10^-5)/(1 ⋅ 10^-1) = x
x = approx. 2 ⋅ 10^-4 (actual: 1.90 ⋅ 10^-4)
pH = -log([H3O+])
pH = -log(2 ⋅ 10^-4)
pH = 3.8 (actual: 3.72)
Need help with MCAT math? Become an MCAT math wizard using Andrew's High-speed Math Mastery Course @ https://mcatselfprep.com/course/andrews-high-speed-math-mastery-course/
A buffer solution contains which of the following?
(A) A strong acid and its conjugate base
(B) A strong acids and its conjugate acid
(C) A weak acid and its conjugate base
(D) A weak acid and its conjugate acid
(C) A weak acid and its conjugate base
A buffer solution contains a weak acid and its conjugate base.
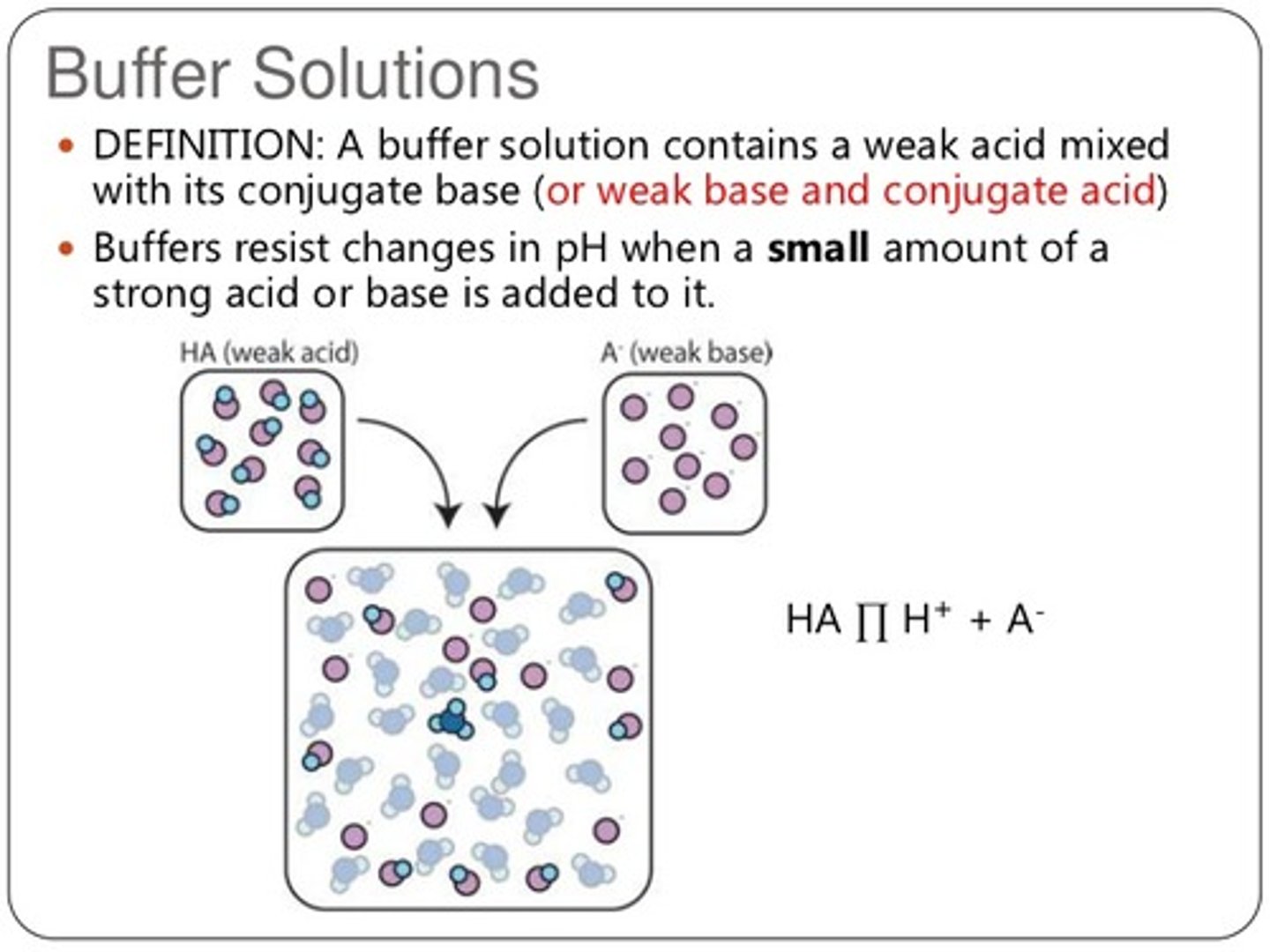
Explain how a weak acid and its conjugate base make for a good buffer solution?
A weak acid will react with OH-, removing it from the solution. Its conjugate base will react with H+, removing it from the solution. This effectively protects our solution from an increase or decrease in the pH.
But why not use a strong acid and its conjugate base?
The conjugate base of a strong acid is EXTREMELY weak and as such would not react with H+, leaving your solution vulnerable to decreases in pH.
What is the purpose of the Henderson-Hasselbach equation?
To help us calculate the pH of a buffer solution that contains varying amounts of acid and conjugate base (or a base and its conjugate acid).
What is the Henderson-Hasselbach equation?
Struggling to keep your MCAT equations straight? Simply conquer the 100 most important equations using Andrew's 100 Most Essential Equations Mastery Course @ https://mcatselfprep.com/course/andrews-equation-mastery-course/
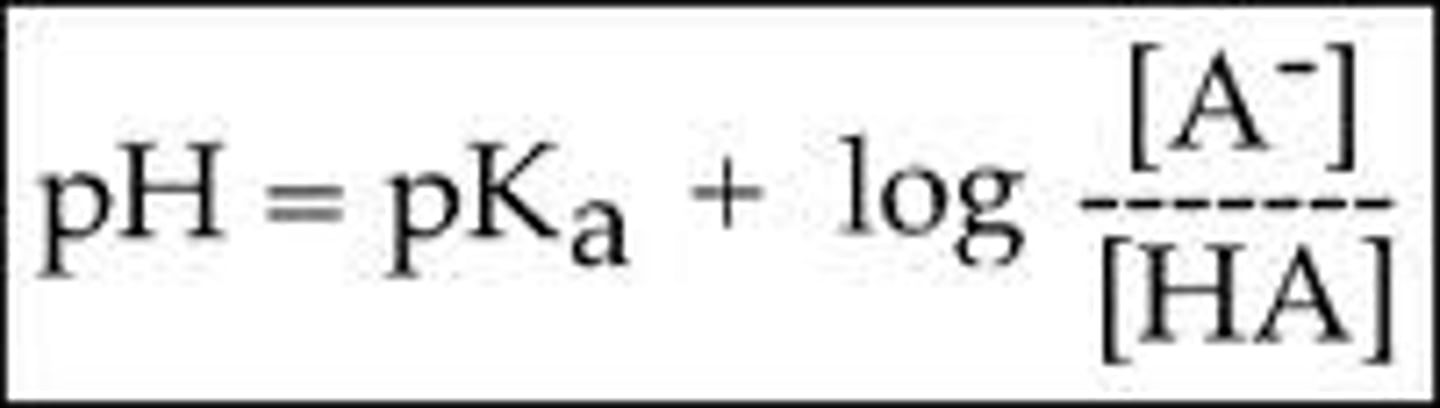
CRB True or false? A buffer has its maximum buffering capacity when its pH is greater than its pKa.
False. A buffer has its maximum buffering capacity when its pH is EQUAL to its pKa.
What is the pH of a solution that contains equal concentrations of its conjugate acid and its conjugate base (pKa = 5)?
(A) 4
(B) 5
(C) 6
(D) 7
(B) 5
The pH equals the pKa when the concentrations of acid and conjugate base are equal. You did not need the HH equation to solve this. :)
You have a buffer solution containing a weak acid (pKa = 4.2) that has a pH of 7. A base is added, increasing the OH- concentration of the buffer solution by 10x the original amount. What is the new pH?
(A) -3
(B) 6
(C) 8
(D) 17
(C) 8
Because we are told that the hydroxyl concentration of the solution has increased tenfold, we do not need to worry about the buffering capabilities.
You can use the Henderson-Hasselbach equation to solve this type of problem, but you should be able to solve it conceptually just knowing that the pH scale is a logarithmic one.
Need help with MCAT math? Become an MCAT math wizard using Andrew's High-speed Math Mastery Course @ https://mcatselfprep.com/course/andrews-high-speed-math-mastery-course/
What is the pH of a buffer solution that contains 3.3 ⋅ 10^-5 M NH3 and 7.2 ⋅ 10^-5 M NH4+ (Ka = 5.6 ⋅ 10^-10)?
(A) 7.56
(B) 8.32
(C) 9.02
(D) 10.67
(C) 9.02
Don't even waste your time pulling out the Henderson-Hasselbach equation for this one! The concentrations of the conjugate acid and base are less than a factor of ten apart and there is more acid than base, so the pH must be slightly less than the pKa (9.25). Most buffer solution questions on the MCAT should be solved intuitively without using the HH equation! :)
Need help with MCAT math? Become an MCAT math wizard using Andrew's High-speed Math Mastery Course @ https://mcatselfprep.com/course/andrews-high-speed-math-mastery-course/