Carbonyls and carboxylic acids
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
38 Terms
What are aldehydes and ketones?
organic compounds that both contain the carbonyl functional group C=O
Describe aldehydes
(include where the carbonyl functional group is and how aldehydes are written in structural formula)
carbonyl functional group found at the end of the carbon chain. Carbon atom attached to 1 or 2 hydrogen atoms
structural formula aldehyde written as CHO
Describe ketones
(include where the carbonyl functional group is and how aldehydes are written in structural formula)
carbonyl functional group joined to 2 C atoms in carbon chain. In its structural formula ketone written as CO
How can aldehydes be used to make carboxylic acids?
they can be oxidised to make COOH when refluxed with acidified dichromate ions (usually mixture of sodium/potassium dichromate and dilute sulfuric acid)
Do ketones undergo oxidation reactions
no
What influences the reactivity of aldehydes and ketones?
the nature of the C=O bond as the double bond is made up of both a pi and sigma bond
the C=O bond is polar
How is the C=O bond pola?
oxygen = more electronegative than carbon
electron density in double bond lied closer to oxygen = carbon = delta positive + oxygen delta negative
Due to the polarity of the C=O bond what can aldehydes and ketones react with? (explain
some nucleophiles
nucleophile attracted to + attacks the delta postive C resulting in addition across C=O double bond
what is the difference between pi bonds in alkenes and pi bonds in carbonyls?
pi bond in alkenes = non - polar + electrophilic addition
pi bond in cabonyles = polar + nucleophilic addition
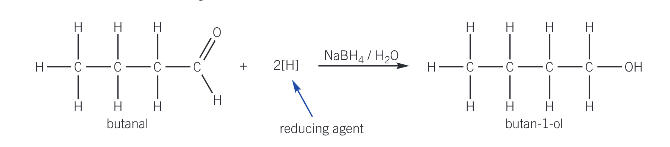
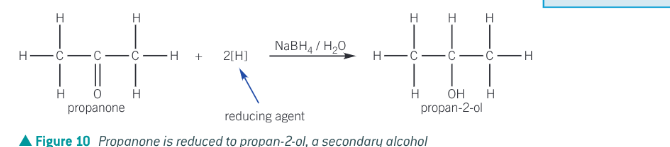
What is used as a reducing agent to reduce aldehydes and ketones?
NaBH4 - sodium tetrahydridoborate
aldehyde/ketone usually warmed with NaBH4 reducing agent in aq solution
What are the aldehydes and ketones reduced to?
aldehyde - primary alchols
ketones - secondary alcohols
formula for aldehydes reduced to primary alcohols by NaBH4
formula for ketones reduced to secondary alcohols by NaBH4
What is HCN (hydrogen cyanide) and can it be used in an open lab?
a colourless, extremely poisonous liquid that boils slightly above room temp so cannot be used in an open lab
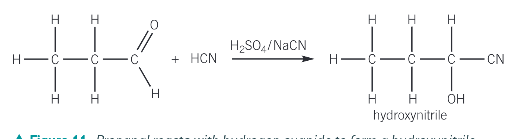
What is the addition reaction with HCN and why is it useful?
It is useful because it provides a means of increasing the length of the carbon chain
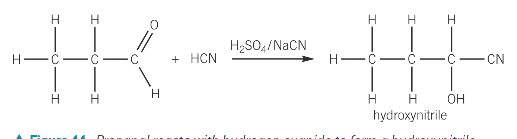
Because HCN is extremely poisonous and hazardous what is used to produce HCN in the addition reaction of aldehydes and ketones
Sodium cyanide and sulfuric acid
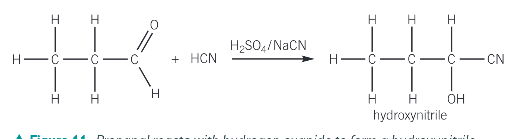
What functional groups does the product of the reaction of HCN with ketones and aldehydes produce? What is the name of the prouct?
a hydroxyl group -OH
a nitrile group -C≡N
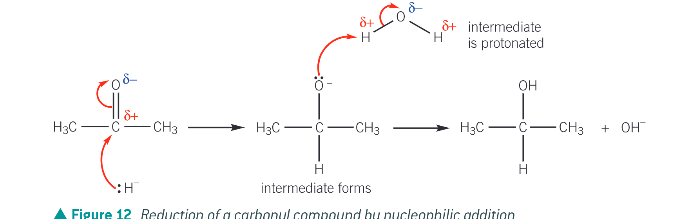
Describe the mechanism for the nucleophilic addition of aldehydes and ketones (with NaBH4)
NaBH4 contains the hydride ion which acts as the nucleophile. Its lone pair of electrons is attracted and donated to the electron deficient delta positive C of the C=O bond
a dative colaveltn bond is formed between hydride ion and C (of C=O)
the pi bond in C=O breaks by hetrolytic fission forming a negatively charged intermediate
oxygen atom of intermediate donates lone paire of electrons to hydrogen atom in a water molecule. Intermediate has then been protonated to form an alcohol
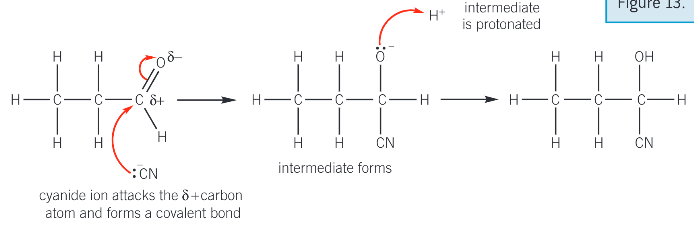
Describe the mechanism for the reaction of NaCN/H+ with an aldehyde or ketone
the cynanyde ion attacks the electron deficient C. The lone pair from the cyanide ion is attracted and donated to the delta + carbon atom in the aldehyde or ketone C=O double bond. A dative covalent bond forms
The pi bond breaks in C=O by heterolytic fission forming a negatively charged intermediate
the intermediate is protonated by donating a lone pair f electrons to a hydrogen ion to form the product
the product is a hydroxynitrile
(second stage can also be drawn showing protonation by water
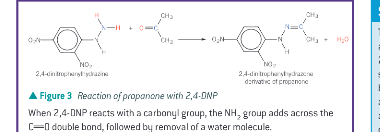
What is CNP/DNPH/Brady’s reagent used to detect? How does it show this?
the presence of a carbonyl functional group.
In the presence of a carbonyl group a yellow or orange precipitate called 2,4-dinitrophenylhydrazone is produced
Why can 2,4-DNP be very hazardous?
because friction or a sudden blow can cause it to explode
How do you test for the carbonyl group in aldehydes and ketones?
Add 5cm depth of 2,4-DNP to a clean test tube - it is in excess
using a dropping pipette, add three drops of unkown compound and leave to stand
if no crystals from, add a few drops of sulfuric acid
a yellow/orange precipitate indicated the presence of aldehyde/ketone
the test tube can then be analysed to identify the aldehyde/ketone
condensation reaction
what is tollen’s reagent?
a solution of silver nitrate in aq ammonia
Once a compound has been identified as containing a carbonyl compound using 2,4-DNP a fresh sample can be further classified as either an aldehyde or ketone using tollens reagent. How do you carry this out (+make the tollens reagent)
in a clean test tube add 3cm depth of aq solver nitrate
add aq sodium hydroxide to the silver nitrate until brown silver oxide precipitate forms
add dilute ammonia solution until brown precipitate just dissolves to form clear colourless solution. Tollen’s reagent
pour 2cm depth of unknown solution into test tube
add equal volume tollens reagent freshly prepared
leave test tube to stand in beaker of warm water around 50degreesC for about 10-15 mins + observe whether silver mirror formed
when tollens reagent reacts with an alddehyde what can be observed
a siver mirror
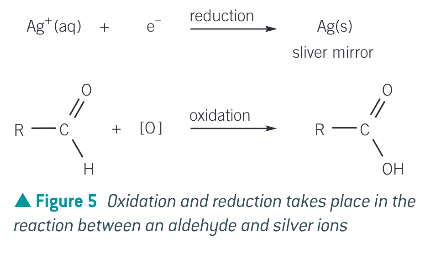
How does tollen’s reagent form a silver mirror in the presence of an aldehyde?
Tollen’s reagent contains silver ions which act as an oxidising agent in the presence of ammonia. In the reaction silver ions are reduced to silver as the aldehyde is oxidised to a carboxylic acid
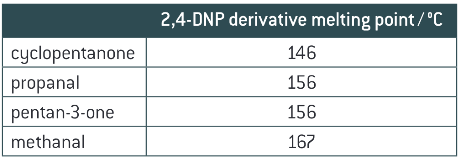
How can you identify an aldehyde or ketone by melting point?
the 2,4-dinitrophenylhydrazone precipitate formed in the 2,4-DNP test can be analysed to identify the carbonyl compound
the impure yellow/orange solid is filtered to separate the solid precipitate from the solution
the solid is then recrystallised to produce a pure sample of crystals
the melting point of the purified 2,4-dinitrophenylhydrazone is measured and recorded
When the melting point of the aldehyde/ketone is measured and recorded how is the carbonyl identified?
the melting point is compared to a database or data table of melting points to identify the original carbonyl compound
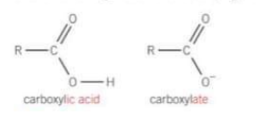
Why are carboxylic acids soluble?
C=O and O-H bonds in COOH are polar allowing carboxylic acids to form H bonds with water but only up to 4 carbons
as the number of carbon atoms increases the solubility decreases as non-polar carbon chain has a greater effect on overall polarity of molecule
True or false carboxylic acids are weak acids
true
What reactions do carboxylic acids take place in and what do they form in these reactions?
redox - with metals
and neutralisation - with bases
they form carboxylate salts
Describe a redox reaction with a carboxylic acid: what does it form, what do you observe
forms hydrogen and carboxylate salt. Observe metal disappearing and effervescence as hydrogen gas evolved
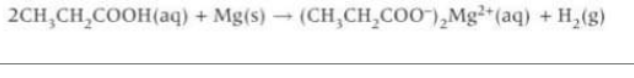
True or false carboxylic acids react like normal acids with bases
true
Is carboxylic acids is in excess and reacts with a carbonate what would you observe happens to the solid carbonate
the solid carbonate would disappear
How can you test for the carbonyl group in a carboxylic acid?
carboxylic acids are the only common organic compounds sufficiently acidic to react with carbonates.
so test with neutralisation reaction with carbonates