Quantum
1/12
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
Explain the nature of electromagnetic radiation
2 different models to describe electromagnetic radiation:
Photon model: how electromagnetic radiation interacts with matter
Wave model: how electromagnetic radiation propagates (spreads out) through space
Photon
A quantum of energy of electromagnetic radiation
Energy of a photon
E = hf
E = hc/wavelength
Electronvolt
A unit of energy (for a tiny amount of energy)
1 eV = 1/6×10(-19) J
Outline how you can determine a value for Planck’s constant by using LEDs
Use the equation eV=hc/wavelength
Work out energy of one emitted photon using E=VQ (because in a LED, the photons of a specific wavelength will be emitted only when the p.d. across them is above a critical value). Measure the critical value voltage here (minimum voltage required to turn on LED)
Plot a graph of V against 1/wavelength. Y=mx+c is equal to V=hc/e(x wavelength) so gradient = hc/e
Use a variety of wavelengths to increase accuracy
Outline a simple method of demonstrating the photoelectric effect
Gold leaf electroscope experiment - shows the PARTICULATE NATURE OF ELECTROMAGNETIC RADIATION.
Briefly touch top of plate with negative electrode from high voltage supply (this charges the electroscope to deposit excess electrons onto the plate. These excess electrons spread down)
Both stem and gold leaf have negative charges so repel eachother
Place clean piece of zinc on the negatively charged electroscope
Sine UV radiation onto zinc surface: gold leaf falls towards stem because electrons lost so negative charge lost)
Explain the photoelectric effect using ideas of photons, conservation of energy and work function.
one photon interacts with one electron
Einstein’s photoelectric equation: hf= ø - KEmax
Ø (aka the work function) is the minimum energy required to remove one electron, so electron is removed when energy of photon is greater than work function
KEmax of photoelectron is independent of the intensity of the incident radiation
Rate of emissions of photoelectrons above the threshold frequency is directly proportional to the intensity of the incident radiation
Outline how we can observe electron diffraction - this PROVIDES EVIDENCE FOR WAVE-LIKE NATURE OF ELECTROMAGNETIC RADIATION
Electron gun fires electrons at a thin piece of polycrystalline graphite. (The gaps between the C atoms are x10(-10)m = similar to electron wavelength so electrons are diffracted. Electrons behave like:
Particles (accelerated by p.d.)
Waves (diffracted)
Particles (hit screen w/ discrete impacts)
What evidence do we have for electromagnetic radition’s wave-like nature and particle-like nature?
Wave-like: diffraction when electron gun shoots electrons through polycrystalline graphite
Particle-like: the photoelectric effect: (only emitted if above threshold frequency, instantaneous emission - not built up energy, increasing intensity did not increase kinetic energy of emitted photoelectrons - only increasing frequency past threshold frequency increases KE)
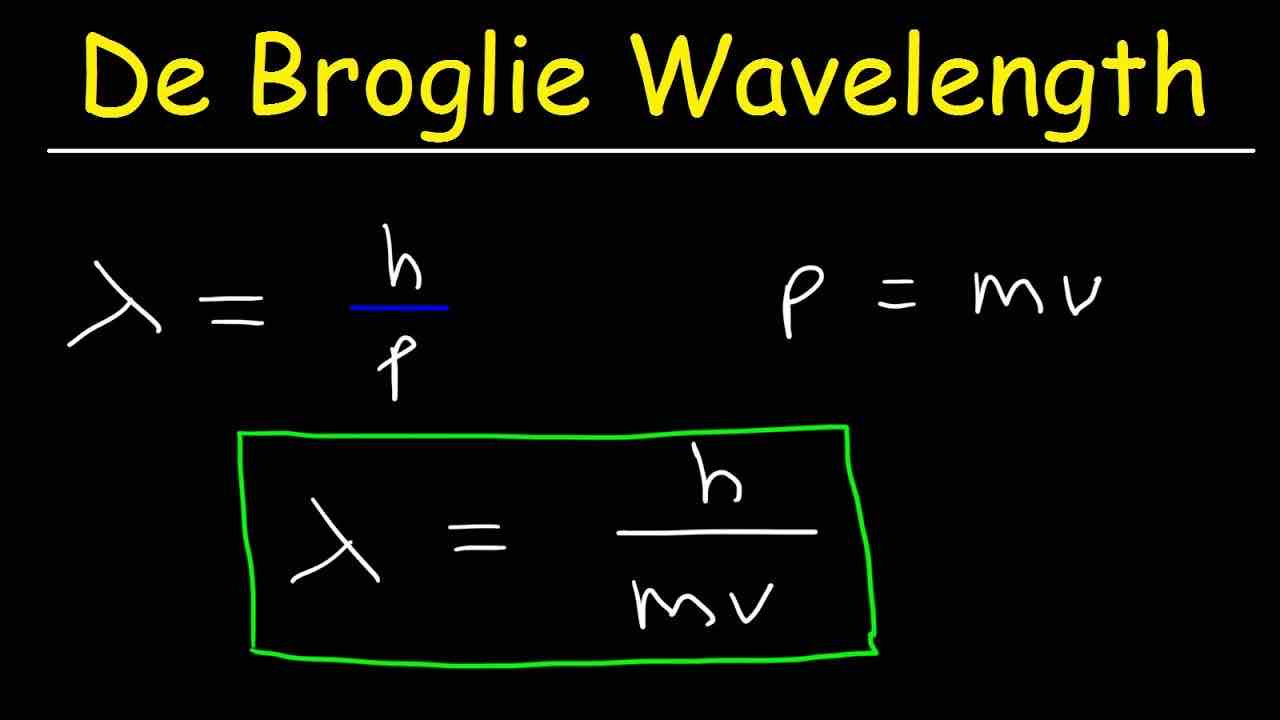
What does the De Broglie Wavelength show?
It allows us to work out the wavelength of an electron. Every partciles has wave nature, but it is only truly evident when the particle is very light (such as an electron which is 9.11×10(-28)g)
Define hf in terms of Eintsein’s photoelectric equation
hf is the energy of the photon (because hf= work function + KEmax)
The wavelength of the incident radiation on a photocell is kept constant but the intensity of the radiation is doubled. State and explain the effect, if any, on the current in the photocell. (2 marks)
the rate of photons incident of M is doubled
The rate of emission of photoelectrons is doubled so the current doubles
The potential difference in an electron gun is increased. Explain how the diffraction pattern will change. (2 marks)
kinetic energy increases
diameter of rings decreases and becomes brighter