4.1.2-Alkanes and Alkenes
1/64
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
65 Terms
what is an alkane
saturated hydrocarbon containing C-C and C-H bonds as sigma (σ) bonds
sigma bonding in alkanes
caused by overlap of orbitals directly between the two atoms
free rotation around the σ bond
general formula of alkanes
CnH2n+2
how many bonds around each carbon?
what is the bond shape around each carbon?
4 σ bonds
tetrahedral geometry → bond angle 109.5° to minimise electron-electron repulsion between bonding pairs
factors affecting boiling point of alkanes
chain length
branching
how does chain length affect bp of alkanes
as chain length increases for straight chain alkenes, boiling point increases
larger SA of contact=greater London forces=more energy required to overcome the London forces in order to separate molecules
how does branching affect bp of alkanes
branched chains have lower boiling points than their straight chain counterparts
Less SA of contact between molecules=less London forces= less energy required to separate molecules
reactivity of alkanes
unreactive→ C-C and C-H are strong
bonds are non polar→ small difference in electronegativity
complete combustion of alkanes
in presence of sufficient oxygen
alkane + oxygen → carbon dioxide + water
incomplete combustion of alkanes+ risks
in presence of insufficient oxygen
alkane + oxygen → carbon monoxide (or carbon particulates) + water
carbon monoxide is a toxic gas
oxides of nitrogen and sulfur produced as by-product→ acid rain
carbon particulates from unburnt fuel can cause respiratory issues
reactions of alkanes with halogens
don’t react with halogens without UV light
in presence of UV a chain reaction called free radical substitution can occur→ forms haloalkane and hydrogen halide
three stages of free radical substitution
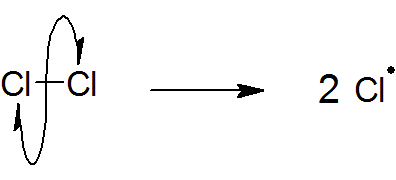
initiation
propagation
termination
initiation
free radicals are formed
UV light required
makes two highly reactive radicals
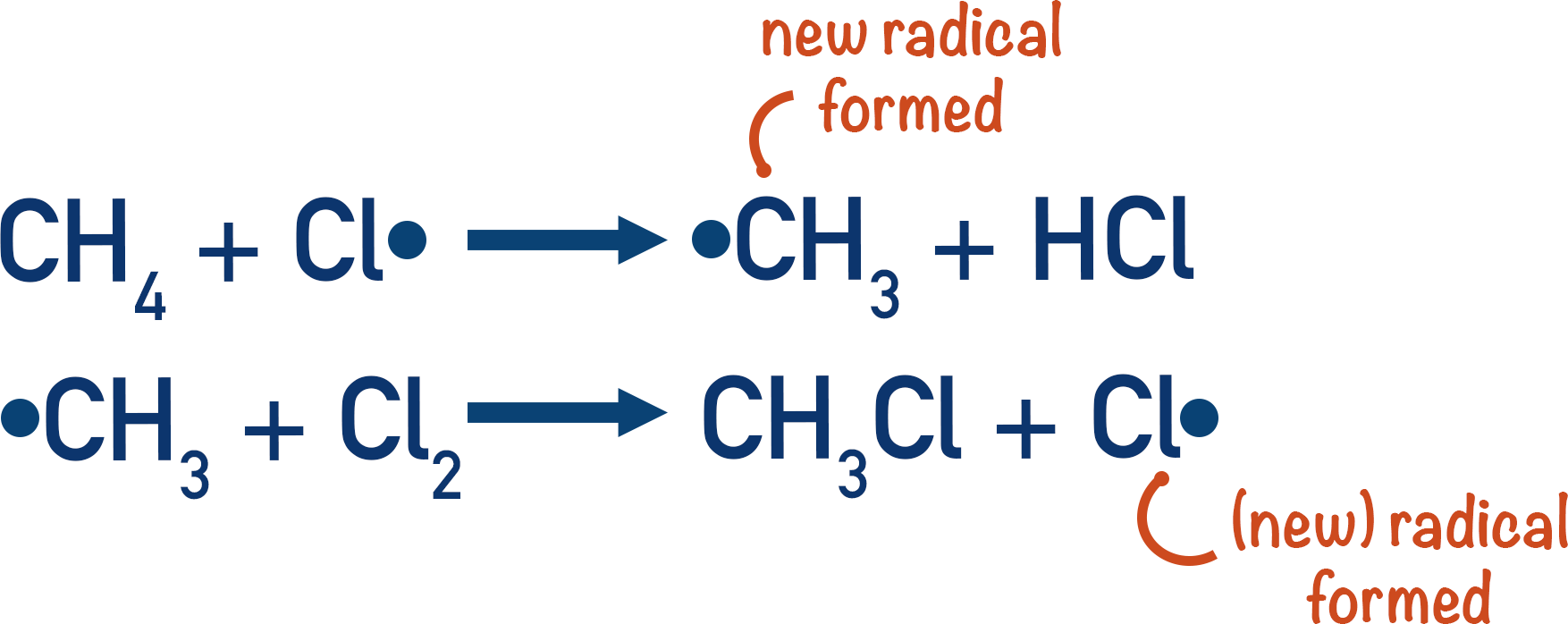
propagation
free radical reacts with the alkane to form more radicals
step 1→ halide radical reacts with alkane to form alkyl radical and hydrogen halide
step 2→ alkyl radical reactions with excess halogen to form haloalkane and halide radical
halide radical must be preserved→ same radical at start and end of propagation
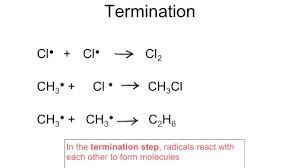
termination
free radicals destroyed→ radicals always join together to form an unreactive substance
repropagation
propagation step can continue many times to result in multiple substitutions→ chain reaction
limitations of free radical substitution
have low atom economy as many products formed in chain reaction
presence of reactive free radicals makes radical substitution unpredictable→ can be formed at any C in the alkane
fractional distillation/chromatography required to purify desired product
Bonding in alkenes
three bonding regions, no lone pairs
trigonal planar
120° bond angle
types of bonding formed in alkenes
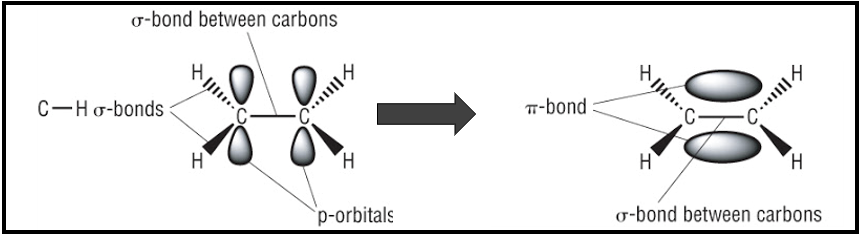
Sigma (σ) bonding
Pi (π) bonding
sigma bonding in alkenes
caused by head on overlap of orbitals
3 of 4 valence electrons are used to form 3 sigma bonds:
1 electron electron forms σ bond with other carbon atom
2 electrons for σ bond with hydrogens bonded to carbon
Pi Bonding in alkenes
caused by the sideways overlap of p orbitals
creates an area of electron density above and below the plane of the carbon atoms
pi bond locks carbon atoms in position→ no free rotation
alkenes general formula
CnH2n
are pi or sigma bonds weaker? Why?
pi bonds are weaker
sideways overlap of orbitals has smaller orbital overlap than head-on overlap of orbitals formed in sigma bonds
less energy required to break pi bonds→ only pi bond breaks when alkenes react
chemical test for alkenes
bromine water
orange/yellow → colourless
criteria for cis/trans isomerism
molecule must have a C=C double bond
each carbon atom must be attached to two different groups
one of the groups must be hydrogen for both carbons
criteria for E/Z isomerism
molecule must have C=C double bond
each carbon atom in the C=C bond must be attached two different groups
Cahn-Ingold-Prelog Priority Rules
assign priority to each group attached to the first carbon- atom with highest Ar has highest priority
Assign priority to each group attached to the second carbon
if two highest priority groups are on the same side→ Z isomer. If on opposite side→ E isomer
what are electrophiles
electron pair acceptors
electron deficient and are attracted to electron rich regions in other molecules such as double bonds in alkenes
Examples of Electrophiles
Hydrogen halides
Halogen molecules
Hydrogen molecules
how are hydrogen molecules and halogen molecules electrophiles
high negative charges of alkene and X2 molecule in close proximity
electron repulsion between electron rich C=C of alkene and electron cloud surrounding X2 molecule
electron cloud in X2 molecule shifts away from electron rich double bond→ induces dipole in X2 with δ+ charge on atom closest to alkene
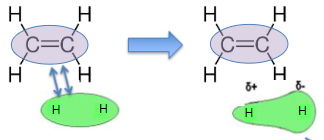
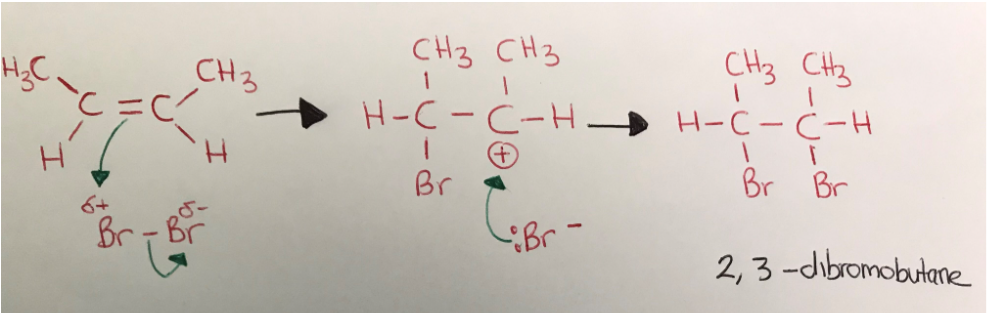
electrophilic addition mechanism (e.g. bromine + but-2-ene)
curly arrows must come from bond in stage one
curly arrow must come from between lone pair in stage 2
hydrogenation of alkenes+ required conditions
hydrogen+alkene→ alkane
150C
nickel catalyst
hydration of alkenes+ required conditions
steam added across double bond
steam + alkene→ alcohol
300°C
phosphoric acid catalyst
stability of carbocations
primary carbocation→ 1 alkyl group bonded to positive carbon atom
secondary carbocation→ 2 alkyl groups bonded to positive carbon atom
tertiary carbocation→ 3 alkyl groups bonded to positive carbon atom
more alkyl groups= more stable
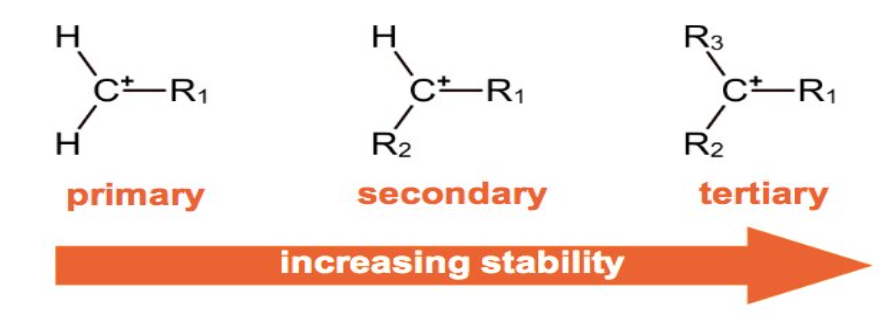
markownikoff’s rule
more stable carbocation→ major product
least stable carbocation→ minor product
types of polymers
biodegradable polymers
bioplastics
photodegradable polymers
biodegradable polymers
type of plastic that can be decomposed by the action of microorganisms and environmental conditions
advantages of biodegradable polymers
used as carrier bags
reused many times
only degrade to carbon dioxide, water and biological compounds
disadvantages of biodegradable polymers
some degrade to leave small pieces of non-biodegradable addition polymers→ harmful to environment
may still require use of non-renewable resources to manufacture
bioplastics
type of biodegradable polymer made from renewable resources e.g. plants starch
advantages of bioplastics
many uses e.g. bin bags, drinks cups, food wraps
decompose to leave no toxic residue
uses renewable resources→ conserves use of non-renewables such as petroleum oil
disadvantages of bioplastics
may create competition for food sources
don’t have long re-use life
photodegradable polymers
type of plastic that is broken down chemically using light energy
advantages of photodegradable polymers
degradation process only requires light
disadvantages of photodegradable polymers
uses non-renewable resources e.g. petroleum oil
may not be exposed to enough light to degrade if in landfill
once exposed to light, begins to break down and not possible to stop process
break down into small particles of plastic rather than breaking down completely
methods of dealing with polymer waste (5)
landfill
combustion
reusing
recycling
feedstock recycling
landfill
rubbish put into large holes in ground and compacted
advantages of landfill usage
methane gas produced→ used for energy generation
new regulations ensure they don’ cause water pollution or damage soil
disadvantages of landfill usage
lots of plastic is non-biodegradable→ can become danger to wildlife
if leak occurred, leachate can cause groundwater pollution
not all methane gas can be collected→ fire risk
no control of what toxic waste ends up in landfill site
combustion
plastics burned/ incinerated in power stations to release energy
advantages of combustion
chemical energy stored in plastics can be transferred to heat energy when burned→ used to drive turbines to generate electricity
disadvantages of combustion
burning plastics e.g. PVC can produce toxic gases e.g. HCl
produces CO2 → greenhouse gas
reusing
some plastics can be reused for same function many times e.g. drinks bottles
advantages of reusing
helps to conserve non renewable resources
reduces amount of waste going into landfill
reduces production of greenhouse gases
disadvantages of reusing
studies show repeated reuse of plastic bottles increases chance that chemicals will leach out of cracks and crevices in containers
recycling
plastics can be melted down and reshaped into new products
advantages of recycling
helps conserve non-renewable resources
reduces amount of waste going into landfill
reduces greenhouse gas production
reduces energy consumption→ less energy required to recycle plastics than to manufacture from raw material
disadvantages of recycling
expensive technology required
not all polymers easily recycled
heat energy required to melt plastics comes from non-renewable resources
melting some plastics can cause release of volatile organic compounds
recycled plastics are often of a lower quality
feedstock recycling
chemical reactions used to break down plastics into small molecules→ used as raw materials to produce new plastics
advantages of feedstock recycling
able to handle unsorted and unwashed polymers
helps conserve non-renewable resources
disadvantages of feedstock recycling
high investment costs involved in the process
what is a polymer
long molecule made of many smaller monomers
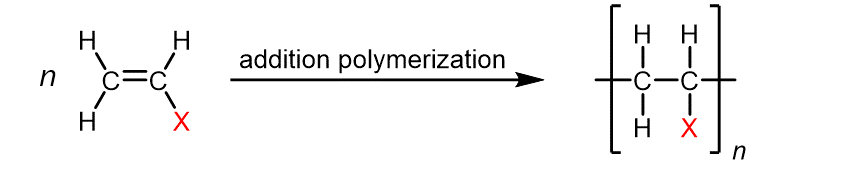
addition polymerisation of alkenes
process when alkene monomers are added to form long chains
LDPE
contains branched chains:
low density
low melting point
prevents polymer packing tightly together→ reduced London forces
HDPE
more straight chains:
high density
high melting point
allows polymer to pack tightly together→ increased London forces