Orgo chp. 2 (families of Carbon compounds)
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
Alkenes
contain C double bond C
prone to addition rxns
Sp2
Alkanes
contain C single bond C
not reactive
Sp3
Families of hydrocarbons (C-H only)
Propane: An alkane with only single bonds and is saturated
Propene: An alkene containing double bonds and is not saturated
Cyclopropane: cyclic alkane (ring structure) with cyclic prefix and -ane ending. Unsaturated with two H per ring
Propyne: at least 1 triple bond and unsaturated with 4 H per tb
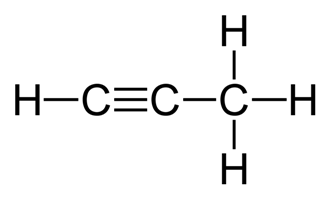
Benzene
Cyclic compound with double bonds
one H per C
Huckle number of electrons
Huckle number
4n+2
n = electrons within pie bond
should equate to total electrons occupying pie bonds in a structure
Resonance in benzene
Benzene has two resonance structures
You can show delocalization (charge spread evenly across molecule) by drawing a circle within the ring structure
6 delocalized electrons increasing stability
Alkanes are named according to number of carbons in chain
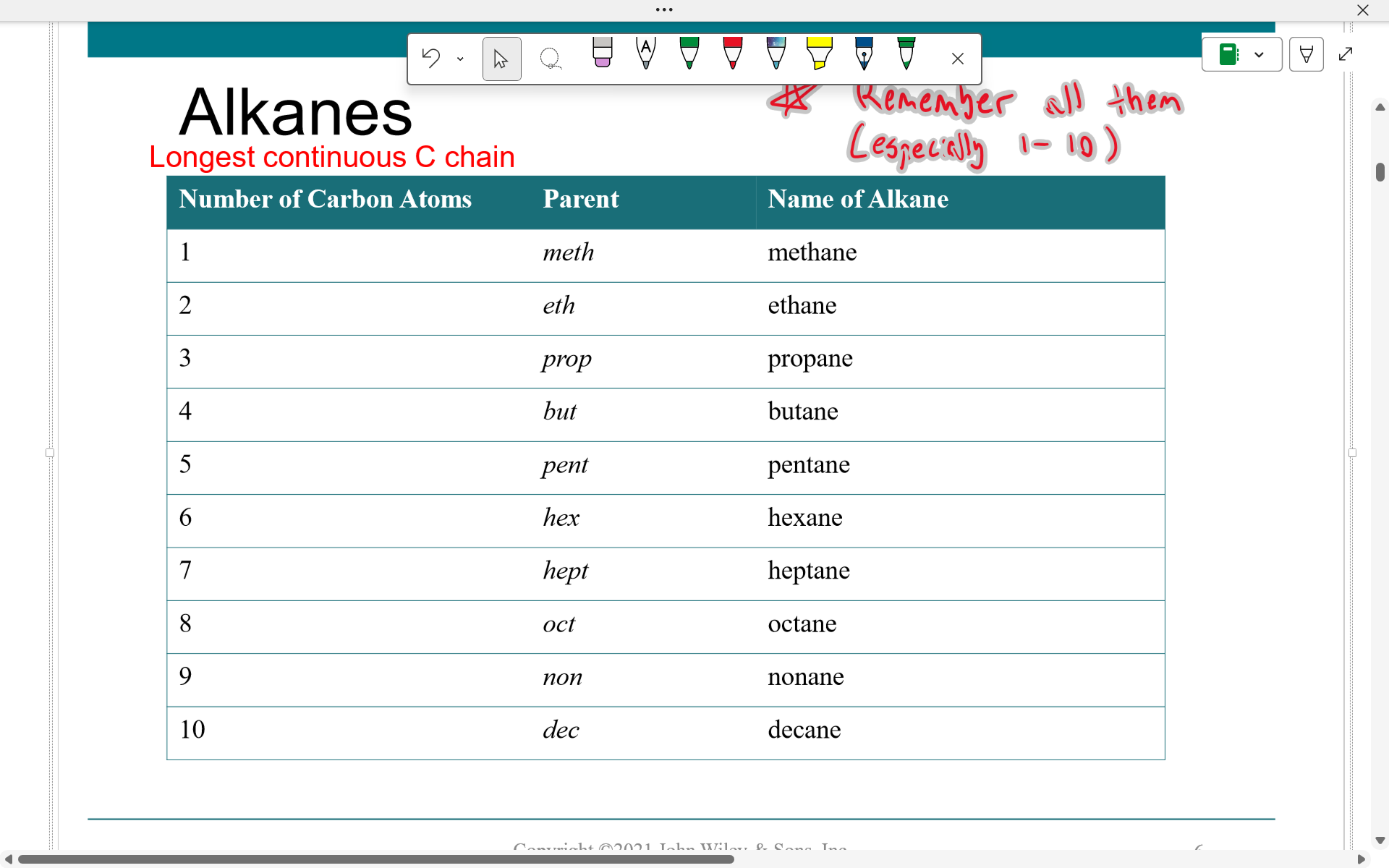
R representation of alkyl groups
By removing one H from an alkane you switch the -ane ending to -yl
alkane —> alkyl
Aryl group
any aromatic ring structure minus one hydrogen
ex.
phnyl (C6H5—)
—CH2—
methylene group that can be attached to phenyl group
Haloalkanes or alkyl halide
Primary alkyl halide: halogen bonded to first carbon
Secondary alkyl halide: halogen bonded to second carbon
tertiary alkyl halide: halogen bonded to third carbon
Other halogen compounds
alkenyl (alkene) halide: halogen bonded to alkene DB
phenyl halide: halogen bonded to aromatic ring group
Alcohol functional group
ROH
hydroxyl group attached to sp3 hybridized C
Classification of types of alcohols
Primary alcohol: OH attached to 1st carbon (benzyl alcohol or ethyl alcohol)
Secondary alcohol: OH attached to 2nd carbon (isopropyl alcohol)
tertiary alcohol: OH attached to 3rd carbon (tert-butyl alcohol)
Phenols (different from phenyls)
OH is directly attached to aromatic ring (Thymol)