Module 2 - OCR A Level Biology
1/160
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
161 Terms
Magnification
The number of times larger an image appears compared to the size of the object
Magnification calculation
Objective lens x eyepiece lens
Image size / actual size
Using stage graticule
insert eyepiece graticule into x10 eyepiece
place stage graticule on microscope stage and bring into focus using low power objective (total mag=40)
align eyepiece graticule and stage graticule. Check value of one eyepiece division at this mag
1mm = 40 eyepiece divisions
each eyepiece divison 1000/40 = 25
use x10 objective lens on microscope and focus on stage graticule
align them both
100 eyepiece divisions now correspond 1mm
1000/100 = 10
Nucleus
Control centre of cell
Stores organisms genome
Transmits genetic information
Nuclear envelope
Separates contents of nucleus from the rest of the cell
Nucleolus
Found inside the nucleus and produces ribosomes
RER
Ribosomes attached
Large surface area
SER
Synthesis cholesterol
Synthesis lipids/phospholipids
Synthesis steroids hormones
Golgi apparatus
Stack membrane bound flattened sacs
Processes and packages proteins
Mitochondria
Site of ATP production during aerobic respiration
Fluid filled matrix
Folded into cristae
Chloroplasts
Site of photosynthesis
Plant cells
Stacks flattened membranes (thylakoids)
Vacuole
Filled with water and solutes
Maintain cell stability
Lysosomes
Engulf old organelles and foreign matter then digest
Formed from Golgi apparatus
Cilia
Beat and move mucus
Ribosomes
Synthesise proteins
Small spherical
Attached to RER
Centrioles
Cell organelle that aids in cell division in animal cells only
Formation cilia
Two bundles microtubules right angle
Cytoskeleton
A network of fibers that holds the cell together, helps the cell to keep its shape, and aids in movement
Cellulose cell wall
The rigid cell wall which surrounds plant cells
How insulin is made
mRNA copy of instructions for insulin made in nucleus
mRNA leaves nucleus through nuclear pore
mRNA attached to a ribosome on RER leads to assemble protein (insulin)
insulin molecules pinched off in vesicles travel towards Golgi apparatus
vesicles fuse with Golgi apparatus
process and package insulin molecules ready for release
insulin pinched off in vesicles move towards plasma membrane
vesicles fuses
exocytosis
Similarities of Prokaryotes and Eukaryotes
Plasma membrane
Cytoplasm
Ribosomes
DNA
RNA
Differences of Prokaryotes and Eukaryotes
Smaller
Less developed cytoskeleton
No nucleus
No membrane bound organelles
Peptidoglycan
Smaller ribosomes
Naked DNA
Flagella
Pili
binary fission
A form of asexual reproduction in which one cell divides to form two identical cells.
covalent bond
A chemical bond that involves sharing a pair of electrons between atoms in a molecule
condensation reaction
Two molecules join with the removal of water
hydrolysis reaction
Two molecules split by addition of water
hydrogen bond
Weak interaction
Slightly negative and slightly positive charge (delta neg/pos)
water as a liquid
provide habitats major component of tissues in living organisms medium for chemical reactions effective transport medium
density of water
more dense as it gets colder up until 4 degrees then due to polarity will become less dense stable environment insulate bodies of water
water as a solvent
positive and negative parts of water attracted to positive and negative parts of solute molecules and ions move around and react transport whilst in water
cohesion and surface tension of water
hydrogen bonding between molecules pulls them together (cohesion) surface of water contracts to resist forces applied (surface tension) xylem pond skaters
high specific heat capacity of water
need a lot of heat energy to increase KE and temperature amount of energy needed to raise 1KG substance by 1 degree stable environment for enzyme controlled reactions aquatic animals
high latent heat capacity of water
helps molecules break away from each other to become a gas large amount of energy needed for evaporation cool things and keep temperature stable
water as a reactant
photosynthesis hydrolysis digestion synthesis large molecules
carbohydrates
carbon, oxygen, hydrogen 1:1:2
use of carbohydrates
energy source energy store structural units
monosaccharides
simplest form carbohydrates source of energy soluble in water insoluble in solutes straight chains or ring
Monosaccharide examples
glucose, fructose, galactose
disaccharide
two monosaccharides joined by a glycosidic bond soluble sweet can hydrolyse
Disaccharide examples
alpha glucose + alpha glucose = maltose
alpha glucose + fructose = sucrose
alpha glucose + beta glucose = lactose
beta glucose + beta glucose = cellulose
polysaccharides
polymers of monosaccharides
homopolysaccharide or heteropolysaccharide
Polysaccharide examples
amylose, amylopectin, glycogen
Amylose
coils into spiral
alpha glucose molecules
1-4 glycosidic bonds
less soluble
unbranched
Amylopectin
coils into spiral shape
alpha glucose molecules
1-4 and 1-6 glycosidic bonds
branched
glycogen
in mammals smaller chains, less tendency to coil 1-4 and 1-6 glycosidic bonds
branched more compacted (easier to snip off)
Cellulose
plant cell walls
long chains
15,000 beta glucose molecules
condensation reaction
1-4 glycosidic bonds
second molecule turned 180 degrees
microfibrils
60-70 cellulose cell walls joined
10-30 nm in diameter
bundle in to macrofribrils embedded in pectins
plant cell wall structure and function
cellulose macrofibrils run in all directions
glycosidic and hydrogen bonds provide high tensile strength
hard to digest
fully permeable
can be reinforced with other substances
bacteria cell wall
peptidoglycan
exoskeleton cell walls
chitin
lipids
group of substances soluble in alcohol rather than water
examples of lipids
triglycerides, phospholipids, steroids
Trigylcerides
glycerol + 3 fatty acids condensation reaction ester bonds
Saturated triglycerides
No double bonds
Tend to be solid at room temp
unsaturated triglycerides
double bonds
liquid at room temp
Phosopholipids
phosphate + two fatty acids condensation reaction
hydrophilic head, hydrophobic tail
micelle phospholipid bilayer (cholesterol)
Cholesterol
steroid alcohol lipid
4 carbon based rings
hydrophobic molecule
regulates fluidity of membrane
amino acids
N-C-C amino group
r group
carboxyl group
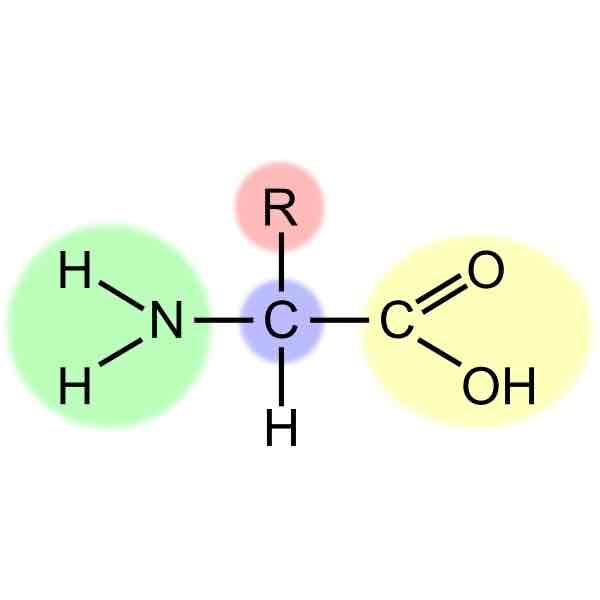
dipeptide
Two amino acids bonded together
peptide bond (polypeptide)
primary structure
sequence of amino acids
hydrogen bonds
secondary structure
Either an alpha helix or a beta-pleated sheet
hydrogen bond
tertiary structure
precise shape
supercoiled or spherical
hydrogen bonds, ionic bonds, disulfide bridges
quaternary structure
Results from two or more polypeptide subunits
hydrogen bonds, ionic bonds, disulfide bridges
fibrous proteins
regular, repetitive sequences
amino acids insoluble form fibres
structural function
fibrous proteins examples
collagen (mechanical strength)
keratin (strong eg. hair, nails, horns)
elastin (skin stretch around bones)
globular proteins
spherical shape
hydrophobic R groups turned inwards, hydrophilic R groups turned outwards
soluble
specific shapes
roles as enzymes, hormones, haemoglobin
globular protein examples
haemoglobin
insulin
pepsin (digests protein in stomach)
inorganic ions
cations (Ca, Na , K, H )
anions (NO, HCO, Cl, OH)
Test for Carbohydrates (Starch)
add iodine solution
blue-black
test for carbohydrates (reducing sugars)
benedict's test
place sample of food in boiling tube
add benedicts solution then heat in water baths at 80 degrees for 3 mins
orange-red precipitate
test for carbohydrates (non-reducing sugars)
test sample for reducing sugars
take separate sample and boil in hydrochloric acid to hydrolyse sucrose and fructose
cool solution and use sodium hydrogencarbonate ions to neutralise
test for reducing sugars again
green-yellow-orange-red
testing for lipids
emulsion test
mix sample with ethanol
filter
pour into clean test tube
cloudy white
testing for proteins
Biuret test
lilac
Chromatography
wear eye protection
draw line in pencil
put dot on line to show where to place solution
spot mixture onto the pencil dot line using a capillary tube
wait for spot to dry before putting another
cover beaker with watch glass
let apparatus run until solvent has reached point just below top
TLC plate lay on white tile till dry
Rf value equation
Distance travelled by substance / distance travelled by solvent
Nucleotide
A building block of DNA, consisting of a five-carbon sugar covalently bonded to a nitrogenous base and a phosphate group
Structure of DNA
polymer of nucleotides
antiparallel (5’ to 3’/3’ to 5’)
adenine, cytosine, thymine, guanine
phosphodiester bond
long
encode genetic information
Purines
Adenine and Guanine
Bases with a double-ring structure
Pyrimidines
Cytosine and Thymine
Bases with a single-ring structure
antiparallel sugar-phosphate backbone
5 end is where phosphate group attached to 5th carbon of deoxyribose (left)
3 end is where phosphate group is attached to 3rd carbon of deoxyribose (right)
very stable
semi-conservative replication
in each new DNA double helix, one strand is from the original molecule, and one strand is new
DNA replication steps
helix unwinds
helicase enzyme unzips
free phosphorylated nucleotides in nucleoplasm bonded to exposed bases
dna polymerase catalyses addition new nucleotide bases in 5 and 3 direction to single strands of dna using it as a template
leading strand is synthesised continuously and the lagging strand is in fragments
later joined by ligase
hydrolysis activated nucleotides to release extra phosphate makes phosphodiester bond
RNA
ribose
uracil
single strand
shorter chain
mRNA, tRNA, rRNA
genetic code
universal: all organisms have same
triplet DNA bases code for same amino acids
degenerate: for all amino acids there are more than one base triplet
non-overlapping: read starting at fixed point in groups of three bases
transcription
gene unwinds and unzips
H bonds between complementary nucleotide bases break
RNA polymerase catalyses the formation of temporary H bonds between complementary nucleotides and unpaired bases (A-U)
the length RNA complementary to template strand of gene is produced, coding strand
mRNA passes out the nucleus through envelope and attaches to ribosome
Translation
tRNA brings amino acids and find their place when anticodon binds by temp H bonds to complementary codon on mRNA molecule
ribosome moves along length mRNA when two amino acids adjacent peptide bond forms
energy in form ATP needed for polypeptide synthesis
after assembled mRNA breaks down and can be recycled into new lengths
chaperone proteins fold protein into 3D shape
Active site of an enzyme
the region of an enzyme that attaches to a substrate
Intracellular enzymes
work inside the cell where they control cell metabolism (eg respiration)
catabolic: metabolites broken down into smaller
anabolic: synthesise larger molecules
Extracellular enzymes
enzymes that act outside of the cell in which they are produced
amylase, trypsin
prosthetic group
A non-protein, but organic, molecule (such as vitamin) that is covalently bound to an enzyme as part of the active site, e.g. zinc + carbonic anhydrase
Cofactors
Any nonprotein molecule or ion that is required for the proper functioning of an enzyme
Cofactors can be permanently bound to the active site or may bind loosely with the substrate during catalysis
coenzyme
organic cofactor
B12, folic acid, B3, B6, B1
lock and key hypothesis
The idea that enzymes are specifically shaped to fit only one type of substrate
induced fit hypothesis
Theory of enzyme catalysis which states that the partial binding of a substrate to an enzyme alters the structure of the enzyme so that its active site becomes complementary to the structure of the substrate, enabling binding.
Enzymes and temperature
gain KE
increase collisions
ES complexes increase
optimum
vibration break H bonds
breaks tertiary structure
denatured
Rate of reaction equation
1 / time taken to reach end point
temperature coefficient (Q10)
a measure of how much the rate of a reaction increases with a 10 °C temperature increase.
enzymes and PH
H bonds hold structures like alpha helix in place as H+ increases the positive charges are attracted to the negative charges so replace the H bonds
work at narrow range PH
buffers
resists changes in PH can donate or accept H+ e.g. haemoglobin
Enzymes and substrate concentration
increased substrate conc leads to increased ROR so substrate conc limiting factor
all enzymes present at max rate so no longer limiting factor
all active sites activated
enzymes and enzyme concentration
enzyme concentration is limiting factor
substrate concentration is limiting factor
fixed conc