NEUROINFLAMMATION
1/56
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
57 Terms
Give a brief overview of the immune system of the periphery
Innate immunity
two lines if defence
skin & mucous membranes (physical barriers)
Internal innate immune response (rapid non-specific)
Adaptive immunity
mainly antibody mediated to destroy or suppress the pathogen (slow and specific with memory)
Give examples of the first line of defence in the innate immune system of the periphery
skin and hair - physical barriers to microbes
mucous membranes - secrete mucous to trap and kill microbes
bodily functions to expel microbes - e.g. coughing and sneezing
chemical - gastric secretions, tears, perspiration to trap, kill or disable pathogens
Describe the second line of defence in the innate immune system of the periphery
Occurs after foreign invaders break through the first line of defence
Releases:
Interferons (Interfere with viral replication)
Complement (help to recognise, destruct and clear pathogen)
Iron binding proteins (reduces iron available for bacterial growth
Antimicrobial proteins
….THEN
NK cells
Non-specific - attack any cell that is ‘unusual’ or ‘abnormal’
Release perforin to cause cell to rupture
Macrophages
Phagocytose
Antigen presenting cells for adaptive immune system (keep foreign fragments on cell surface for recognition)
Describe adaptive immunity in the periphery
Antigen-specific responses, includes T and B lymphocytes.
Defence against specific invaders
Bacteria, toxins, viruses
T cells primarily focus on recognizing and killing infected cells
B cells are responsible for producing antibodies that target and neutralize foreign invaders
How does adaptive immunity differ from innate immunity in the periphery?
Adaptive immunity has:
Specificity
Memory
Describe the central immune system and its components
Central Immune System (Brain)
The brain is immune-specialized and lacks traditional adaptive immunity.
It is separated from the periphery by the blood-brain barrier (BBB).
Dominated by microglia (80%)
Other identified immune cells are include;
myeloid cells
monocytes/macrophages
dendritic cell
lymphocytes (T-cells, B-cells, natural killer (NK) cells) - relatively limited presence
Where are lymphocytes found in the brain?
most are found in the choroid plexus and meninges
also in some parts of the brain parenchyma
Describe the development of microglia
Microglia develop in utero and mature post-natally
derived from yolk sack and get to brain via bloodstream
Describe the physical barriers of the central immune system
The barrier systems protect the brain from invading pathogens by forming a tight barrier which is difficult to penetrate – this includes vascular endothelial cells.
Meninges: Protective layers surrounding the brain and spinal cord.
Blood-Brain Barrier (BBB):
Formed by tight junctions between endothelial cells (and transmembrane proteins) of brain capillaries.
Prevents entry of large molecules and immune cells from the bloodstream.
Vascular endothelial cells:
Line blood vessels of the CNS, with extensive tight junctions
Regulate immune cell trafficking across the BBB (impermeable to most substances).
Describe microglia and their role in the central immune system
Microglia are macrophage-like cells making up 80% of CIS
Resident immune cells of the CNS.
Constantly monitor the brain environment.
Respond rapidly to injury/infection by phagocytosis and cytokine release.
Perform functionally important tasks in the brain:
phagocytosis of foreign agents
synaptic pruning
Brain protection and repair
Describe astrocytes and their role in the central immune system
Astrocytes are glial cells with differing morphologies and functional characteristics found in a variety of brain areas that support neurons.
Help maintain the BBB, regulate neurogenesis and synaptogenesis
Release chemical mediators in response to inflammatory conditions
List the three key inflammatory mediators
complement
cytokines and chemokines
pro-inflammatory and anti-inflammatory factors
Describe the complement system
Are a series of (~40) plasma proteins that enhance immune responses, including the ability of antibodies and phagocytes to clear pathogens and damaged cells
Stimulates inflammation, phagocytosis, opsonization, and direct cell lysis
Three pathways of complement activation, all of which involve C3b complement
Very important in neuroinflammation - CNS injury and infection lead to increased complement protein expression.
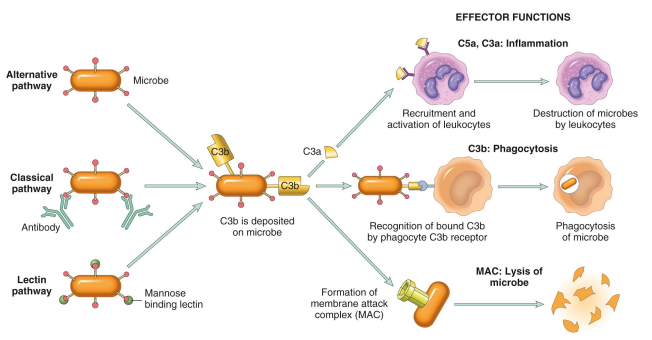
Describe the 3 pathways of complement activation
The three pathways of complement activation are the classical pathway, the lectin pathway, and the alternative pathway, each leading to the activation of C3 and promoting inflammation and opsonization.
Classical pathway:
Triggered by: Antibodies bound to antigens
Leads to: Recognition and phagocytosis of microbe (C3b)
Alternative pathway:
Triggered by: direct recognition of microbe
Leads to: Inflammation - recruitment and activation of leukocytes, destructing the microbe
Lectin pathway:
Triggered by: Mannose-binding lectin (MBL)
Forms membrane attack complex (MAC)
Leads to: Lysis of the microbe (by the MAC)
Briefly describe cytokines and chemokines
Cytokines are signalling proteins that act as allow cells to communicate with each other, playing a key role in helping the host respond to infection, immune cell signalling, and inflammation
Chemokines are a subset of cytokines with the specialized function of directing immune cells to sites of infection or inflammation
Cytokines are responded to by a large number of cells (functional redundancy and pleiotropism)
Describe a situation in which cytokines can be detrimental
The initial neuroinflammatory response can be beneficial, but long-term chronic overproduction of cytokines is detrimental and can fuel the degenerative process.
How are cytokines produced?
Cytokines are synthesized by immune cells in the periphery and by glial cells and other brain resident cells in the CNS.
List three types of cytokines
interleukins
tumour necrosis factors
growth factors
Describe interleukins
Interleukins are critical messengers that regulate both innate and adaptive immunity.
They can be pro-inflammatory (like IL-1, IL-6, IL-17) or anti-inflammatory (like IL-10).
Their balance is key in preventing overactive immune responses (e.g., autoimmunity) or immune suppression.
Describe the key types of interleukins
Each play crucial roles in immune response regulation.
Key types of interleukins include:
pro-inflammatory IL-1, IL-6, IL-17,
anti-inflammatory IL-10,
IL-18 (produced by microglia and astrocytes) further activates other microglia
Describe tumour necrosis factor
Tumour Necrosis Factors (especially TNF-α) are a family of cytokines involved in inflammation, immune regulation, and cell death.
produced by microglia and astrocytes in response to injury
receptors are TNFR1 and TNFR2
activate microglia and astrocytes, regulate BBB permeability and promotes inflammation
Contributes to neuroinflammation in diseases like Alzheimer’s, stroke, multiple sclerosis.
Describe growth factors
Growth factors are signaling proteins that regulate cell growth, disease and repair
TGF-β, is the main one and plays important roles such as regulation of neuroinflammation, and apoptosis in traumatic brain injury
Describe chemokines
A sub-type of cytokines that guides cell movement
Expression is generally modulated by pro-inflammatory stimuli.
Functions:
Stimulate chemotaxis lymphocytes to sites of injury, damage, or infection
Regulate leukocyte infiltration across the BBB during inflammation and infectious diseases
Classified into 4 main subfamilies based on the position of 2 of their 4 conserved cysteine residues near their N-terminus:
C
CC
CXC
CX3C
Describe how the anti-inflammatory response is dictated
The inflammatory response is dictated by a balance of pro-inflammatory and anti-inflammatory factors.
Each opposing pathway is mediated by different cytokines and certain hormones.
Pro-inflammatory Factors (mediate): IL-1b, IL-6, IL-17, and TNF-a.
Anti-inflammatory Factors (inhibit): IL-10 and TGF-b.
Chronic inflammation occurs when this balance is perturbed.
Describe the activation of microglia associated with neuroinflammation
Microglia is constantly surveying the brain, when inflammation occurs, they transition from surveillance to activated state (M1 phenotype).
Immunogenic factors at high concentrations cause microglia to undergo morphological and chemical changes, including the production and release of cytokines and reactive oxygen species (ROS).
This response is regulated by the type and severity of the insult/injury.
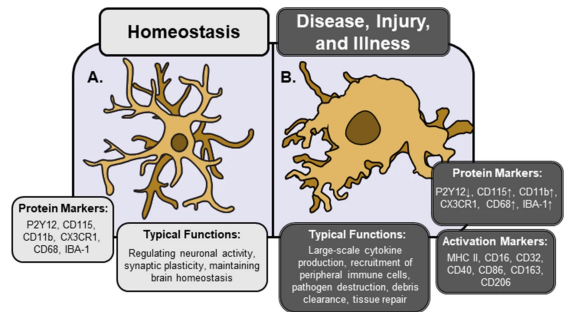
Describe the activation of astrocytes associated with neuroinflammation
Astrocytes can mount diverse responses to promote CNS repair or pathology.
It is not an all-or-none process and differs depending on context.
A common feature is the upregulation of glial fibrillary acidic protein (GFAP), in response to most types of CNS injury, widely used as a marker of astrocyte reactivity.
Describe signalling that occurs from the activation of astrocytes in neuroinflammation
Upon release of cytokines by microglia, astrocytes release a variety of signalling molecules which can modulate neuronal function, promote repair processes, or exacerbate inflammation
The transcription factor NF-κB plays a central role in regulating the pro-inflammatory response of astrocytes.
NF-κB activation is triggered by cytokines released by microglia and other CNS cells during inflammation, as well as by AHR in response to environmental signals
Once activated, NF-κB moves into the nucleus and promotes the transcription of inflammatory genes, such as those encoding IL-6.
This results in the release of pro-inflammatory cytokines or, in some cases, activation of cell death pathways.
Describe cross-talk that occurs from the activation of astrocytes in neuroinflammation
Communication between astrocytes and microglia regulates their responses to neuroinflammation (A).
Activated astrocytes release factors such as TNF to drive apoptosis in oligodendrocytes.
Further communication occurs through the release of cytokines (B).
Astrocytes can also recruit leukocytes into perivascular spaces and CNS parenchyma through the secretion of certain factors (C).
Interactions between activated astrocytes (releasing CCL2) and endothelial cells increase BBB permeability and facilitate leukocyte infiltration.
Describe the infiltration of monocytes and macrophages that can occur in neuroinflammation
During neuroinflammation there is also a breakdown of the BBB and this allows circulating inflammatory factors and immune cells to enter the brain parenchyma.
In response to BBB breakdown or infection:
Glia activation causes the release of pro-inflammatory cytokines, which break down the BBB.
This leads to the infiltration of inflammatory cells, mainly neutrophils.
Activated neutrophils release pro-inflammatory cytokines, which cause further glia activation leading to neuronal injury.
Describe the differences between activation of microglia and astrocytes
Microglia primarily act as the immune cells of the CNS, responding rapidly to injury through phagocytosis and cytokine release, while astrocytes support neuronal health by maintaining homeostasis and modulating inflammation.
Activation of microglia is more associated with acute responses to injury, whereas astrocytes play a role in both repair and chronic inflammatory states.
Give examples of neuroinflammatory diseases
Stroke and Ischemic Events
Oxygen deprivation which leads to neuronal death.
Ischemic stroke results from permanent or transient occlusion of a major brain artery or one of its branches.
Associated with a massive activation of microglia and astrocytes
Neurodegenerative Diseases
Alzheimer’s Disease: Chronic microglial activation, amyloid plaque-associated inflammation, activating microglia.
Parkinson’s Disease: Inflammation contributes to dopaminergic neuron degeneration in substantia nigra.
Infectious Agents
Viral, bacterial, fungal, and parasitic infections can induce neuroinflammation
What happens to microglia in the hours and days following a stroke?
They become ameboid and take on an M1 phenotype
List the brain areas critical for cardiovascular control
Circumventricular organs
Highly vascularized structures around the 3rd and 4th ventricles, characterized by the absence of a blood-brain barrier (BBB) e.g. SFO
Brainstem circuits
Crucial for autonomic regulation: NTS receives input from baroreceptors sends to RLVM; integrates cardiovascular signals
Describe how neuroinflammation might influence cardiovascular function
Neuroinflammation can lead to elevated cytokines that modify blood pressure regulation, potentially contributing to hypertension.
Pro-inflammatory mediators (cytokines) are increased in hypertensive animals compared to normotensive animals.
Activated microglia indicate neuroinflammation.
Activated microglia in the PVN are decreased in spontaneously hypertensive rats after reconstitution with normotensive bone marrow.
Describe the proposed mechanism for the impact of neuroinflammation on hypertension
Proposed Mechanism for Neuroinflammation's Impact on Hypertension
Pro-hypertensive signals like Ang II activate PVN pre-autonomic neurons, increasing sympathetic nerve activity (SNA) and causing the release of chemokine ligand 2 (CCL2).
Increased SNA affects the bone marrow (BM), resulting in an increase in inflammatory cells (IC) and a decrease in angiogenic progenitor cells (APCs).
This imbalance is associated with vascular pathology and an increase in blood pressure.
Inflammatory progenitors migrate to the PVN, where they differentiate into BM-derived microglia/macrophages which are activated and release cytokines and chemokines, that causes an increase in sympathetic nerve activity (increasing blood pressure and hypertension)
Describe some of the key inflammatory mediators involved in signalling in CV control circuits
Key inflammatory mediators involved in cardiovascular control include cytokines like TNF-α, IL-1β, IL-6, and CCL2, which affect neural signalling and vascular function. These mediators alter the activity of autonomic circuits, contributing to dysregulation of blood pressure and overall cardiovascular health.
What is the effect of TNF-α on cardiovascular function in the SFO and AP?
Increased TNF-α in the SFO causes a rise in renal sympathetic nerve activity, blood pressure and heart rate
What are the effects of IL-1β on cardiovascular function in the SFO?
Increased IL-1β into the SFO elicits a rise in renal sympathetic nerve activity, blood pressure and heart rate (comparable to TNF-α)
Describe the proposed mechanism for AngII and neuroinflammation
Primary (neurogenic) and secondary (renal stenosis, diet) causes increase circulating AngII levels.
Increase in AngII causes increases in circulating CCL2 (MCP-1).
Causes changes in the brain (disruption of the BBB, infiltration of immune cells, activation of microglia).
Immune cells and activated microglia release inflammatory factors including IL-6, TNF-α, IL-1β, and CCL2.
Exacerbates neuroinflammation, disrupts homeostasis, and activates premotor neurons.
Leads to an increase in SNA and hypertension.
Describe the link between gut microbiota, neuroinflammation and hypertension
Gut microbiota influences neuroinflammation through metabolites and immune signalling, which can lead to increased systemic inflammation and hypertension.
Host microbiota constantly control maturation and fuction of microglia in the CNS
Mechanisms:
Enteroendocrine cell release of gut hormones.
Cytokine release from mucosal immune cells.
Release of bacterial products such as SCFA, GABA, or 5-HT precursors.
Afferent neural pathways, including the vagus nerve.
Stress hormones (NA) might influence bacterial gene expression; signalling between bacteria might change microbial composition.
Describe the four factors that cause the relationship between gut microbiota, neuroinflammation and hypertension
Increased Gut Permeability):
Allows bacterial products into circulation
LPS promotes systemic and central inflammation
Circulating Factors:
Inflammatory signals cross into the brain via CVOs or affect endothelial function
Activate CNS regions (e.g., PVN, SFO), increasing sympathetic outflow
Short-Chain Fatty Acids (SCFAs):
Produced by healthy gut microbiota
Reduces microglia cells stress
Anti-inflammatory and blood pressure-lowering
CNS Signalling Changes:
Neuroinflammation alters autonomic circuits
Sustains elevated blood pressure
Describe the proposed mechanism for the relationship between gut microbiota and hypertension
Hypertension leads to microglia activation.
Drives an increase in SNA from areas such as the PVN
This drives a change in gut microbiota and increased gut permeability.
Results in oxidative stress, changes in microbial products, stimulation of inflammatory cells, and release of cytokines.
Feeds back to potentially further exacerbate neuroinflammation and hypertension.
What is the clinical definition of multiple sclerosis?
Multiple sclerosis is a demyelinating autoimmune disease of the central nervous system.
Myelin is damaged by our own nervous system due to dysregulation
List some symptoms of multiple sclerosis
Symptoms vary between people with MS depending on the location of lesions
MS is heterogenous, so symptoms vary:
Cognitive changes
Muscle weakness or spasms
Difficulty walking and with balance
Numbness and fatigue
Blurred vision
Dizziness
Incontinence, gastrointestinal disruptions
Dysphagia (difficulty swallowing, slurred speech)
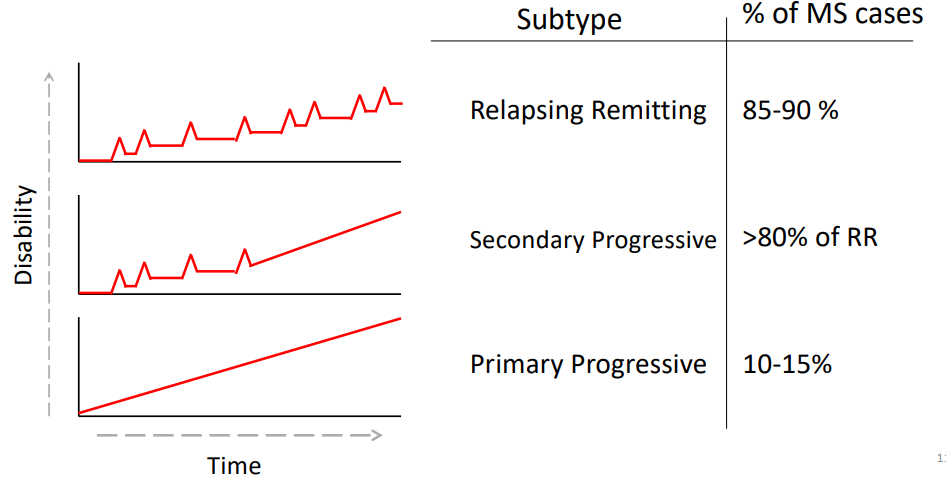
What are the three clinical subtypes of MS?
Relapsing Remitting (85-90% of MS cases)
Characterised by periods of time with increased disease severity, followed by periods of time with reduced symptoms
Secondary Progressive (>80% of RR)
Changes from relapsing to a steady increase in disease severity over time
Primary Progressive (10-15%)
Steady increase in disease severity over time from onset
How do you diagnose MS?
Notoriously difficult to diagnose - previously relied primarily on clinical observations, now use specialized tests and clinical observations
Key Feature of Multiple Sclerosis
Dissemination in space: Lesions occur in different parts of the CNS
Dissemination in time: Lesions accumulate over time
Two main diagnostic tests:
MRI
Oligoclonal bands in cerebral spinal fluid
Indicates ongoing inflammatory response in the CNS
Describe two characteristics of MS
MS is characterised by multiple Lesions at Multiple Sites
People with MS develop decreases in brain volume due to significant atrophy of the brain.
What are the 6 key risk factors of MS?
Caused by a complex interplay of environment and genetic factors including:
Geographical Location
Prevalence increases with distance from equator
hypothesis is levels of vitamin D
Race
more prevalent in Caucasians
Infection
Epstein Barr Virus
Age
20-40 years
Sex
Females are 3x more likely
Lifestyle Factors
smoking, obesity
Genetic Susceptibility
Identify and define the important immune cells involved in the pathogenesis of MS
T cells:
Cytotoxic T cells
Directly interacts with and kills other cells
in the case of MS, oligodendrocytes (maladaptive autoimmune response)
Helper T cells
Direct the immune response of other immune cells
in the case of MS create pathological pro-inflammatory environments in the CNS, recuriting more inflammatory cells
Helper T cells that are pathogenic in MS:
IFN-y
TNF-a
IL-17A
GM-CSF
B cells:
Antibody producing cells
in MS produce antibodies against self-antigens such as myelin leading to damage
Cells of the innate immune system:
Antigen presenting cells such as monocytes, microglia, macrophages and myeloid dendritic cells are key pathogenic cells in MS
These cells activate/reactivate T cells, produce inflammatory mediators and phagocytose myelin
Describe the pathogenesis of MS
We still don't know what the initial step of MS pathogenesis is but what we do know:
In the periphery, antigen presenting cells such as dendritic cells encounter self antigens and migrate to the lymph nodes where they present these antigens to the helper T cell, activating them
The helper T cells migrate through the blood, across the BBB into the CNS where they initiate an immune response after being reactivated by the microglia
This immune response involves the recruitment of other cell types from the blood including B cells, more helper T cells, monocytes and cytotoxic T cells
This leads to a local inflammatory response, including:
the release of proinflammatory cytokines
direct damage via cytotoxic T cells
oxidative damage due to ROS (reactive oxygen species) and RNS (reactive nitrogen species)
Direct phagocytosis of myelin by macrophages and microglia
Antibody and complement mediated damage from B cells
This inflammatory environment results in:
Oligodendrocyte death
Damage to healthy myelinated neurons as well as demyelinated neurons
Neuron and axon loss.
Identify and describe the three types of lesions found in MS
Active lesions:
Seen early
Lots of inflammatory infiltration (BBB permeable)
Astrocyte activation
Demyelinating or post-demyelinating
Inactive lesions:
Not many inflammatory cells (hypocellular
Axon loss
Glial scar (formed by astrocytes)
Mixed active/inactive lesions:
Fewer cells, still some inflammatory cells and astrocytes present
Hypocellular centre, with edges showing ongoing inflammation
Demyelinating or post-demyelinating. Axon loss
Describe the mechanisms of CNS damage
There are many mechanisms, all of which lead to myelin damage and oligodendrocyte death, leaving the axon vulnerable to damage
Mechanisms:
Cytotoxic T cells can directly interact with and kill oligodendrocytes through the release of toxic compounds
The helper T cells release proinflammatory cytokines that can cause damage and stimulate other immune cells
e.g. induce B cells induce antibody and complement mediated damage to both the myelin and oligodendrocytes
can stimulate the innate immune system, activating microglia and macrophages to phagocytose myelin or produce high levels of cytokines (or ROS/RNS), that can damage oligodendrocytes and myelin
The oligodendrocytes are also very sensitive to metabolic dysfunction, where high levels of ROS and RNS can cause disruption of the mitochondria in the oligodendrocytes
Describe three functional consequences of myelin damage
Demyelination disrupts saltatory conduction leading to slowed action potential.
Contributes to MS symptoms
Axons are more vulnerable to damage - leading to axon dysfunction
Remyelination failure also contributes to axon loss
Describe the mechanisms of axon dysfunction
Mechanisms of Axon Dysfunction:
Ion Channel Redistribution
To allow for continuous conduction without saltatory conduction
Inflammatory mediators (e.g. nitric oxide)
Can impair action potential propagation and demyelinated axons are much more sensitive to these
Reversible
Mitochondrial Dysfunction
Impacts the propagation of action potentials
Oxidative damage disrupts mitochondria and prevents them from providing the axon with energy needed to activate the ion channels within the axon
List several MS treatments
Interferon β (IFNβ) - a cytokine
Tysabri - first antibody based treatment
Mechanism of action: Prevents immune cells transiting across blood brain barrier
Binds to the a4 integrins on lymphocytes so it can't bind with VCAM1 so it can't cross
Gilenya - first oral drug
Sphingosine 1-phosphate (S1P) receptor modulator
Prevents immune cells leaving lymphoid organs by preventing them from binding S1P
Ocrevus - B cell deleting therapy
B cell depletion (which eventually recover over ~72 weeks), some T cell depletion (secondary mechanism of action)
Autologous Haematopoietic Stem Cell Translation - immune system reset (most effective but most risky)
Depletion of immune system to remove self-reactive cells
Replace immune system with haematopoietic stem cells
High risk of infection due to lack of autoreactive immune system
What is the problem with current MS treatments?
Current treatments target the immune response and effectively treat relapsing remitting MS
Limited treatment options for progressive MS types
Name several strategies for the the development of remyelinating treatment
Promote OPC differentiation
Remove inhibitors of remyelination
Modulate microglia function
Stem cell therapy