Glycocalyx and Lactate SOTAs
1/177
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
178 Terms
Where is the endothelial glycocalyx located under normal conditions?
Covers the luminal aspect of all blood vessels
What is the endothelial glycocalyx composed of?
Scaffolding of proteoglycans (PGs), glycoproteins (GPs), and glycosaminoglycans (GAGs) associated with the underlying endothelial cells
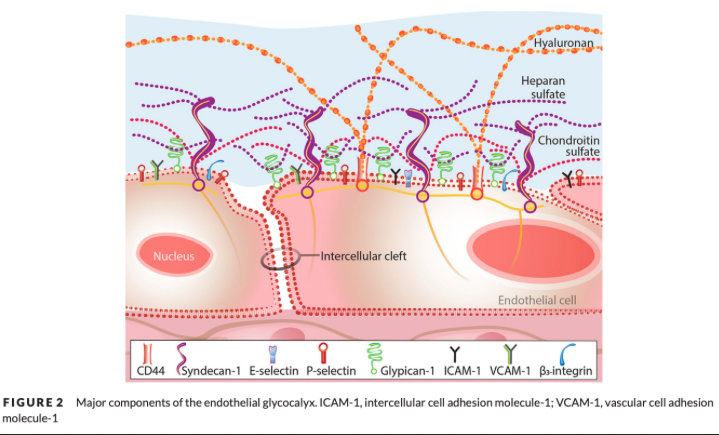
What do the endothelial glycocalyx and its associated molecules and fluid comprise?
The endothelial surface layer (ESL)
What is the function of proteoglycans in the glycocalyx?
Form the main scaffolding
What are the 2 main proteoglycans in the glycocalyx?
Syndecans
Glypicans
What is the function of syndecans in the glycocalyx?
Transmembrane proteins comprised of extracellular, transmembrane, and cytosolic domains
Extracellular domain binds GAGs and detects extracellular signals, such as shear stress, which are transduced to the intracellular environment via the transmembrane portion and the cytoplasmic tail
What is the function of glypicans in the glycocalyx?
Its ectodomain binds only the GAG heparan sulfate (HS)
Its anchor molecule is thought to localize it around lipid rafts and caveolae
Caveolae - membrane structures rich in signaling molecules that serve as communication hubs in the cell membrane
Location allows glypicans to partake in various signaling cascades
What is the most abundant component of the glycocalyx?
Glycosaminoglycans
Form up to 95% of the proteoglycan composition
What are the five main GAGs in the glycocalyx?
Heparan sulfate
Chondroitin sulfate
Dermatan sulfate
Keratin sulfate
Hyaluronan
What is the most abundant GAG in the glycocalyx?
Heparan sulfate
What gives the endothelial glycocalyx a net negative charge?
Sulfate groups attached to GAGs
Synthesis and Attachment of Syndecan and Glypican
Synthesis of core proteins syndecan and glypican occur on membrane bound ribosomes
They are transferred to the lumen of the endoplasmic reticulum followed by the Golgi apparatus where attachment, polymerization, and sulfation of GAG side chains occur
Core protein + GAG transferred to the cell surface where it is incorporated into the cell membrane (syndecans) or attached to the cell surface with an anchor molecule (glypican)
HA is not attached to a core protein and is synthesized on the cell membrane rather than the Golgi apparatus
Glycoproteins of the Glycocalyx
Located on the EC surface
Covered by the EG in health
Endothelial GPs are membrane-bound cell adhesion molecules that are separated into 3 different families based on structural and functional characteristics
What are the families of glycoproteins
Selectins
Immunoglobulins
Integrins
What are selectins involved in?
Initial contact and adhesion of leukocytes and platelets to the activated endothelium
P-Selectin
Found on endothelium and platelets
Constitutively expressed
Stored within granules of Weibel-Palade bodies of EC and platelets
Translocated to the cell surface following stimulation by complement components, thrombin, histamine, or fibrin
Occurs within minutes
E-Selectin
Found exclusively on the endothelium
Inducible
Requires transcription, translation, and translocation to the cell surface
Upregulated following cytokine or antigenic stimulation
Reaches maximal levels within 4-6 hours
Returns to baseline within 24-48 hours
What is the function of immunoglobulins in the glycocalyx?
Support the adhesion and transmigration of leukocytes between ECs
What is the function of integrins in the glycocalyx?
Important for firm attachment of leukocytes and platelets to the endothelial cells as well as transduction of mechanical or chemical signals from the extracellular to the intracellular microenvironment
Endothelial Glycoproteins and the Leukocyte Recruitment Cascade
Presence of endothelial GPs required for functioning of leukocyte recruitment cascade
Rolling
Adhesion
Transmigration
Diapedesis of neutrophils, monocytes, eosinophils, and some lymphocytes is achieved when these adhesion molecules bind to their respective leukocyte integrins
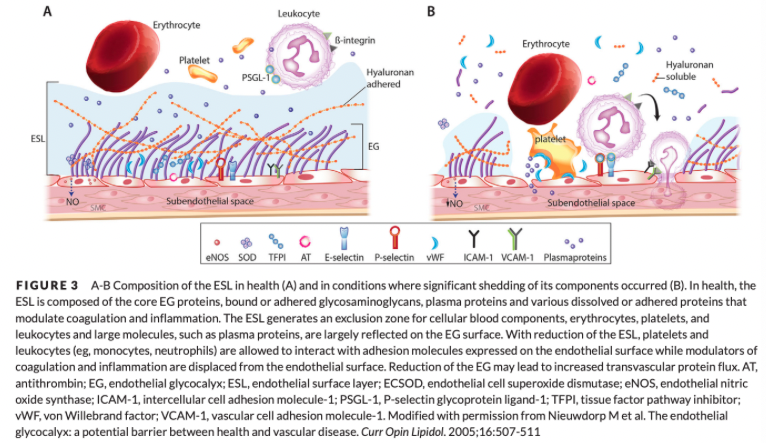
Soluble Components of the Glycocalyx
Soluble plasma components are incorporated into the EG and form the ESL
Enhance the EG by altering its physical properties such as thickness and permeability
Albumin is one of the key soluble components within the EG
Appears to be required to impart normal barrier function to the ESL
Evidence suggests that albumin alone is not sufficient to maintain the barrier function of the ESL
One study found that in addition to charge and size affecting the inclusion of molecules into the ESL, the shape of the molecule also appears to be important
Different GAGs also bind to other molecules, which facilitate their integration into the ESL
Perfused Boundary Region
In health, the negatively charged EG repels RBCs from its surface to facilitate laminar flow
An increase in the perfused boundary region (PBR, area accessible to RBCs within the vasculature) has been shown to be associated with a loss of EG thickness
PBR shown to significantly increase in critically ill people, with septic patients having the largest PBR
What are the crucial roles of the endothelial glycocalyx?
Maintaining normal vascular permeability and transvascular fluid flux
Cell-to-cell interactions (inflammation and coagulation)
Vascular mechanotransduction
Starling Hypothesis
Movement of fluid between the intracapillary and interstitial spaces depends on the osmotic pressure generated by plasma proteins and the hydrostatic pressure (HP) differences between these two compartments
Intravascular fluid is filtered out of the vessel at the arterial end of the capillary and reabsorbed at the venous end
Starling Hypothesis Modified with the Discovery of the Endothelial Glycocalyx
Colloid osmotic pressure (COP) within the interstitium contributes much less to the transendothelial fluid flux than previously thought
Revised hypothesis suggests that the main COP gradient is not the difference of forces between the plasma and the interstitium, but rather between the plasma and the space immediately below the EG, the subglyceal space (SGS)
The very low COP within the SGS causes the net COP difference between the SGS and the capillary lumen to be much larger than the COP difference between the interstitium and the lumen
Possible that the most physiologically relevant COP gradient exists between the ESL and the SGS, which is an even greater gradient than between the capillary lumen and the SGS
Under steady state conditions, the rate of fluid extravasastion is low, but occurs along the entire length of the capillary and without net absorption of fluid at the venous end of the capillary
The COP gradient, which is entirely intravascular, opposes but does not reverse fluid filtration
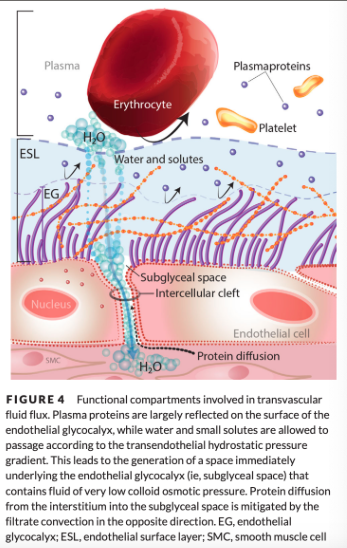
Why is the colloid osmotic pressure so low in the subglyceal space?
Within the SGS, the COP is very low for 2 reasons
Albumin incorporation into the ESL increases its filter function by effectively excluding entry of larger macromolecules
Any back diffusion of proteins from the interstitium into the SGS is prevented by the high velocity of filtered fluid funneled through the interendothelial clefts directionally toward the interstitium
These clefts are the main sites of fluid movement from the vascular lumen to the interstitium
What controls solute exchange? What is passage of solutes restricted based on?
Negatively charged GAGs + the EG's ultrastructure control solute exchange, restricting passage of solutes based on size, shape, and charge
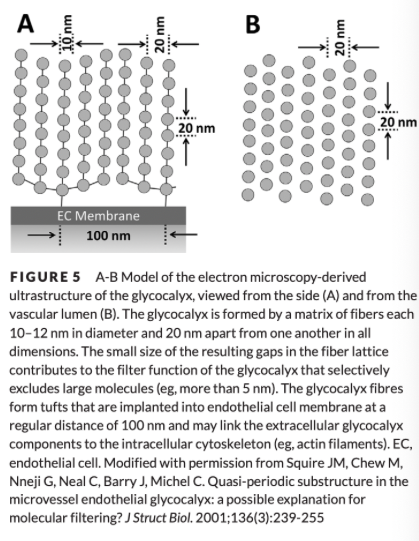
Fiber-Matrix Theory of the Endothelial Glycocalyx Microstructure
a fibrous mesh (the EG) covers the entire endothelial surface and confers the molecular sieving properties to the vessel
Has been experimentally confirmed - enzymatic degradation of the EG resulted in a 60% increase in permeability
What size molecules is the endothelial glycocalyx permeable to?
EG excludes large molecules while maintaining a relative permeability to smaller molecules, specifically those with radii less than 4-5 nm
Gaps of <10 nm significantly restrict permeability of the matrix for larger molecules
Effect of Albumin on the Endothelial Glycocalyx
Albumin alters the EG structure to a regular, lattice-like structure which, in conjunction with its net negative charge, enhances the EGs selectivity to macromolecules
Albumin's effect of reducing vascular permeability is likely not solely due to an increase in COP but also its ability to become incorporated into the EG
Other plasma macromolecules, such as fibrinogen and orsomucoid, are also important to maintain permeability characteristics due to their incorporation into the ESL
Effect of the Glycocalyx on RBC Movement
Thickness of the EG helps maintain normal RBC movement while simultaneously modulating the amount of fluid sheer stress on the EC
EG aids in maintaining the laminar flow pattern (inner region of RBC's surrounded by outer layer of plasma and platelets) by preventing RBC from attaching to the EC
Negatively charged RBC glycocalyx repelled by the negatively charged EG
An intact EG may improve microcirculatory flow by causing RBCs to develop a more elongated conformation which improves the efficiency and speed of RBC transit
Leads to an increase in oxygen exchange capacity and reduction in friction as blood moves through the microcirculation
Changes in EG leads to changes in RBC glycocalyx and vice versa
Endothelial Glycocalyx and WBCs in Health
In health, the GPs responsible for WBC recruitment and initiation of coagulation are hidden beneath the EG so the ESL is anti-inflammatory and anticoagulant
Physical thickness of the EG and its charge prevent circulating WBCs, which have their own glycocalyces, from accessing ECs
EG must be partially shed under inflammatory conditions to allow exposure of EC adhesion molecules and subsequent WBC diapedesis
Shedding of the Glycocalyx and WBCs
Shedding of the EG exposes WBC activators which upregulates WBC integrin expression and potentiates binding to their respective EC adhesion molecules
The Endothelial Glycocalyx and Regulation of Coagulation
In health, the EG regulates coagulation by acting as a physical barrier to prevent EC and platelet adhesion molecule interaction as well as concentrating anticoagulant molecules within its structure
vWF is constitutively expressed on the surface of ECs and hidden beneath the EG, preventing unwanted platelet adhesion and activation
EG also binds many anticoagulant molecules including antithrombin (AT), thrombomodulin [TM], protein C, and TFPI
Endothelial Glycocalyx and Antithrombin
Within the EG, AT binds to regions of heparan sulfate, which enhances its anticoagulant activity on the EC surface
Endothelial Glycocalyx and Thrombomodulin
TM, an integral membrane protein containing chondroitin sulfate, is also constitutively expressed on ECs beneath the EG
Association with chondroitin sulfate is essential to TM's anticoagulant ability and including into the EG
Binding of TM to thrombin and further complex formation with protein C receptor expressed on the EC surface potentiates activation of the protein C anticoagulant pathway
Endothelial Glycocalyx and TFPI
TFPI is bound to heparan sulfate within the EG, inhibits the formation of TF-FVII complex
Mechanotransduction
The transformation of a mechanical force into a biochemical response
Endothelial Glycocalyx and Mechanotransduction
EG core proteins sense shear stress and transmit this signal to the actin cytoskeleton via their transmembrane domain
Under shear stress, ECs produce NO
Production of NO occurs via activation of the enzyme endothelial NO synthase (eNOS), which leads to relaxation of subendothelial smooth muscle cells and thus vasodilation
Degradation of the EG is associated with the loss of shear-induced NO release
Studies suggest that heparan sulfate and/or HA is responsible for sensing vascular shear stress
Without an intact EG, the vasculature cannot appropriately respond to hemodynamic forces, which could lead to direct mechanical damage to the EC and the inability to regulate vascular tone
EG can reorganize its structure under conditions of high flow
Alteration of Vascular Permeability and Transvascular Fluid Flux with Damaged Endothelial Glycocalyx
Damage to the EG can lead to alterations in transvascular fluid movement, capillary leak, and development of edema
The large COP gradient across the EG supports retention of fluid within the vascular lumen
When the EG is damaged, the COP gradient is reduced or lost and fluid movement becomes more dependent on intravascular HP and the transendothelial HP gradient
Destruction of the EG also results in the loss of its molecular sieving properties
Leads to extravasation of large macromolecules into the interstitium, which decreases intravascular COP and increases interstitial COP
In severe vasculitis, the relevant COP gradient is then between the plasma and the interstitial fluid rather than between the plasma and the SGS
Vascular inflammation is associated with the loss of the structure of the EG
Inflamed vessels develop larger gaps in the EG leading to increased permeability
Damaged Endothelial Glycocalyx and WBCs
In health, the EG shields numerous WBC adhesion molecules from circulating cells and creates an anti-inflammatory phenotype
During inflammation, loss or thinning of the EG is physiologically advantageous by exposing the adhesion molecules required for WBC transmigration and ultimately the resolution of infection or tissue injury
With the loss of the EG, the endothelium changes from an anti-inflammatory phenotype to a proinflammatory phenotype
With widespread loss of the EG or with persistent activation of the endothelium, this adaptive response can become detrimental
Excessive WBC adhesion can become detrimental as it physically obstructs the vascular lumen, particularly within microvessels
Causes increased resistance to blood flow and if widespread, microvascular dysfunction can occur
Damaged Glycocalyx and Freely Circulating GAGs
Freely circulating GAGs shed from the EG may also directly affect inflammation in critical illness
Circulating GAGs can bind and impede the action of locally released antimicrobial peptides and activated complement fragments, impairing the body's innate defense mechanisms
Freely circulating GAGs could be associated with a reduced ability to clear infection in sepsis
Circulating GAG fragments may act as local and distant inflammatory stimulators and activate both the innate and adaptive immune systems
How does loss of the endothelial glycocalyx help transition the endothelium from an antithrombotic to a prothrombotic state?
Loss of the EG is a key step in the transition of the endothelium from an antithrombotic to a prothrombotic state that is common in critical illness
Loss of the EG results in an increase in platelet adhesion and markers of hypercoagulability
Increased platelet-endothelial binding triggers EC activation resulting in activation of WBCs and complement
With EG loss the anticoagulant molecules are shed into the systemic circulation
May act at distant sites and contribute to a generalized hypocoagulable state
Damaged Glycocalyx and Mechanotransduction
Loss of the EG leads to a reduction in normal vascular reactivity preventing required alterations in vessel tone
May increase requirement for vasopressor use
Biomarkers of Endothelial Glycocalyx Degradation
Circulating EG degradation products as biomarkers to identify EG alterations or dysfunction are of interest
Matrix metalloproteinase (MMPs) are a family of cell surface proteases that are responsible for degrading extracellular matrices
Thought to be some of the key enzymes responsible for the shedding of the EG in many diseases
Syndecan-1, heparan sulfate, chondroitin sulfate, and HA are all considered valid markers of EG integrity
EG shedding is exaggerated under pathological conditions such as SIRS and sepsis, trauma, ischemia and reperfusion (I-R) injury, hyperglycemia, hypervolemia, and major surgery
SIRS and Sepsis Leading to Glycocalyx Dysfunction
An increase in circulating biomarkers of EG degradation such as SDC1, HS, and HA occurs in animals and people with SIRS or sepsis
Multiple molecules have been identified as possible instigators of EG degradation during sepsis: TNF-a, ROS, MMPs, C-reactive protein (CRP), endogenous catecholamines, and heparanases
TNF-a and Glylcocalyx Dysfunction
Linked to the degradation of the EG in sepsis
Plays at least a partial role in EG degradation during inflammation
During inflammation TNF-a release may activate MMPs and lead to degradation of the EG
C Reactive Protein and Glycocalyx Dysfunction
Commonly used marker of inflammation but also implicated in directly contributing to EG degradation in sepsis
CRP may not only be a marker of inflammation but an active contributor to the associated evolution of vascular dysfunction
Endogenous Catecholamines and Glycocalyx Dysfunction
A large catecholamine surge may be partly responsible for EG degradation in sepsis
In people with naturally occurring sepsis, the level of increase in both catecholamine and SDC1 concentrations was correlated with disease severity
Heparanase and Glycocalyx Dysfunction
An enzyme specific for the cleavage of heparan sulfate
Implicated in the development of ALI in septic patients
Speculated that heparanase expression is induced by ECs after their stimulation by circulating molecules termed "danger signals"
"Danger signals" - endogenous molecules or molecular structures produced or released from cells that are damaged or undergoing cell death or exogenous molecules from pathogens, both of which activate the immune system
Heparanases then partially degrade the EG allowing the exposure of EC leukocyte adhesion molecules to facilitate WBC diapedesis, which ultimately clears the insult
During sepsis, where there is a large concentration of circulating danger signals, there may be diffuse and persistent stimulation of pulmonary heparanase expression, which may lead to widespread pulmonary EG shedding
Systemic Effects of Loss of the Glycocalyx in Sepsis
Development of tissue edema
Identification of high EG biomarkers at presentation to the ICU may be useful to help identify patients at risk of harm from large volume fluid resuscitation and who may benefit from early vasopressor support
Excessive inflammation
Alterations in coagulation
Initial EC activation has been associated with an initial period of hypercoagulability which can later progress to hypocoagulability
Studies have identified an association but not causality between increased EG degradation products and the development of hypocoagulability in sepsis
Reduced vasomotor tone
Using Biomarkers of the Endothelial Glycocalyx to Aid Prognostication in Patients with Sepsis
Multiple studies in people with sepsis have demonstrated an association between increased levels of plasma EG biomarkers and an increase in mortality
Studies have also looked at GAG biomarkers in the urine rather than plasma which found that increased urinary GAG concentrations were associated with higher mortality and morbidity
Ischemia Reperfusion Injury Leading to Glycocalyx Dysfunction
In companion animals, I-R is clinically relevant in the setting of postcardiac arrest (PCA) care and cases of thromboembolic disease
Postcardiac arrest syndrome is associated with the exposure of the body to widespread I-R injury and an increase in EG degradation biomarkers has been demonstrated in this condition
Studies suggest that global I-R may be the cause for EG degradation following cardiac arrest
Increased circulating catecholamine concentrations have also been associated with EG degradation in PCA human patients
The loss of the EG likely contributes to the common sequelae see in these cases: increased vascular permeability, hyperinflammation, coagulopathy, and reduced vascular responsiveness
Trauma and Hemorrhage Leading to Glycocalyx Dysfunction
In people, clinical studies have demonstrated an increase in EG biomarkers, including HS, HA, SDC1, and CS after trauma or hemorrhagic shock
There are no clinical veterinary studies investigating the link between EG degradation and trauma, but animal hemorrhagic shock models have demonstrated EG loss after serious hemorrhage
Suggested mechanism for EG degradation in trauma is related to the large catecholamine release and tissue hypoperfusion that occurs with shock
Hypothesized that this leads to the direct loss of the EG
Studies have found that catecholamine release activates the inflammatory response in trauma and induction of TNF-a may be the mechanism for EG loss
Massive EG loss in trauma is associated with increased vascular permeability, increased systemic inflammation, hypocoagulability, and reduced vascular responsiveness
Glycocalyx Biomarkers and Hypocoagulability in Trauma
A correlation between increased EG biomarkers and hypocoagulability in human trauma patients has been demonstrated, suggesting that EG damage may play a role in acute traumatic coagulopathy
Endogenous Heparinization
GAGs, particularly HS and CS, which are shed after a traumatic insult, act systemically as anticoagulants
Traumatic coagulopathy may represent an adaptive evolutionary response that in some cases becomes maladaptive when severe, unregulated, and widespread or exacerbated by medical interventions such as fluid therapy
Hypothesized that trauma creating an increasingly hypocoagulable state through endogenous heparanization counterbalances the proinflammatory and procoagulant state of an activated endothelium, reducing clot formation and maintaining perfusion through the microcirculation
Shedding of the EG may also be advantageous as it allows for increased vascular permeability and shifting of fluid from the intravascular to extravascular space
Reduces blood pressure, reducing ongoing hemorrhage and shifting of fluid to the extravascular space allows for a pool of fluid for later mobilization
Hypervolemia Leading to Glycocalyx Dysfunction
Hypervolemia leads to atrial distension and release of ANP
ANP reduces intravascular volume by vasodilating vascular beds, increasing renal excretion of fluid, and increasing vascular permeability
ANP release leads to EG shedding resulting in extravasation of fluid and colloids from the vasculature
May lead to detrimental edema formation and reduced oxygenation
Multiple studies in human medicine and veterinary medicine have shown that fluid overload is associated with increased morbidity and mortality
Emphasize that resuscitative fluid therapy is not a benign intervention and call for a judicious and rational approach
Hyperglycemia Leading to Glycocalyx Dysfunction
Transient and chronic hyperglycemia also lead to EG degradation
Findings suggest a link between hyperglycemia-induced EG degradation and an increased risk of cardiovascular disease in patients with diabetes mellitus
Antioxidants to Reduce Glycocalyx Shedding
Protective effects of N-acetylcysteine only present when administered prior to hyperglycemia and before EG loss
Activated Protein C for Reduction in Glycocalyx Loss
Also associated with reduced markers of endothelial oxidative stress, improved microcirculatory function, and improved response to vasopressor therapy in animal endotoxemia models but no mortality benefit found in human studies of severe sepsis and septic shock
Septic people treated with APC had increased risk of developing bleeding complications
Drug is no longer commercially available
Exogenous GAGs to Repair the Glycocalyx
Exogenous GAGs may help reconstitute the EG after shedding but unknown how this may translate to a clinical population
Treatment of people with diabetes mellitus with soludexide for 8 weeks resulted in an increased thickness of the EG compared to controls
Soludexide or administration of other exogenous GAGs would need to thicken the EG and improve its function more quickly than 8 weeks to be clinically relevant to the critical care population
Heparin to Reduce Glycocalyx Shedding
Speculated that unfractionated heparin mobilizes intracellular pools of SDC1, leading to reformation of the EG
Also may inhibit heparanase, which cleaves HS in sepsis
Hypothesized that LMWH binds to components of the EG, such as HS, and inhibits or reduces the release of heparanase from the EC
Colloids to Reconstitute the Glycocalyx
The ESL is the in vivo structure responsible for normal vascular integrity
Treatment with plasma proteins may therefore aid in EG reconstitution
Infusion of 5% human albumin led to a reduction in the extravasation of fluid after I-R injury
Albumin appears to be able to penetrate and bind within the EG, reforming the ESL and restoring vascular integrity
Evidence suggests that synthetic colloids are not superior to crystalloids for the preservation of the EG
Possible that providing albumin to reconstitute the EG in critical illness could help restore microvascular barrier function, but appropriate albumin dose to achieve a beneficial effect remains unknown
Also not possible to know prior to administration whether any EG scaffolding remains to absorb albumin
Risk that administration of endogenous or synthetic colloids could lead to extravasation of the macromolecules into the interstitium, worsening edema
Fresh Frozen Plasma (FFP) for Repairing the Glycocalyx
Animal hemorrhagic shock models have demonstrated that fluid resuscitation with FFP compared to crystalloids successfully restores the EG
Use of FFP leads to improved microhemodynamics, vascular hemostasis, and reduced leukocyte-endothelium interaction compared to crystalloids or synthetic colloids
A possible mechanism is that FFP may restore the structural scaffolding of the EG by replacing SDC1 and preventing its further loss
FFP also contains albumin and other plasma proteins that are important in maintaining the ESL
FFP may be beneficial as a method to restore the ESL
Protease Inhibitors such as AT to Reduce Glycocalyx Shedding
n experimental I-R and sepsis models, treatment with AT reduced EG shedding and attenuated vascular permeability and tissue edema
Multiple possible mechanisms for protective effects of AT
Inhibition of cleaving enzymes such as heparanase at the site of inflammation
Thrombin reduction
Reduction in the amount of heparanase released from mast cells during inflammation
AT found in FFP
Use of AT in critical illness is controversial
Given lack of conclusive evidence for benefit of AT and documented risk for clinically significant hemorrhage, the 2017 Surviving Sepsis Campaign Guidelines recommend against the use of AT in sepsis and septic shock
Doxycycline to Preserve the Glycocalyx
At subantimicrobial doses, doxycycline reduces EG shedding through inhibition of MMPs
Glycocorticoid Administration to Reduce Glycocalyx Shedding
Suggested mechanisms of protective effects
Stabilization of mast cells because their degranulation releases proteases that can degrade the EG
Suppression of MMPs
Currently the Surviving Sepsis Campaign Guidelines do not advocate the use of corticosteroids in sepsis
Lactic Acid at Physiologic pH
At physiologic pH, lactic acid is essentially fully dissociated into lactate anions and protons (H+)
What are the two stereoisomeric forms of lactate?
L-lactate
D-lactate
L-Lactate
In health, accounts for >99% of total body lactate
Isomer of major physiological significance
D-Lactate
Formed either by the glyoxalase pathway or produced by commensal bacterial in the mammalian GI tract and absorbed into circulation
Hyperlactatemia
Serum, plasma, or blood lactate concentration is above the relevant reference interval
Lactic acidosis
Moderate to severe hyperlactatemia with concurrent metabolic acidosis
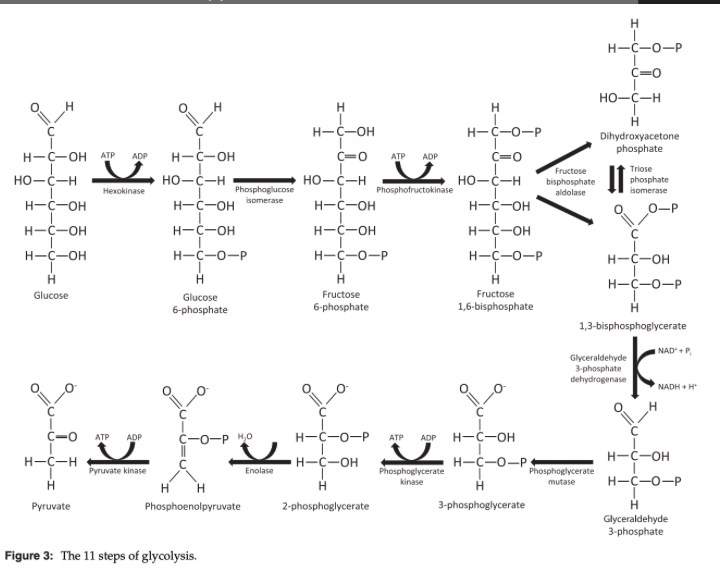
What does glycolysis produce?
3 molecules of pyruvate, 2 molecules of ATP, 2 molecules of NADH
What does glycolysis require to produce ATP?
Constant supply of glucose and NAD+ but does not require oxygen
Glycolysis Steps
Pyruvate after Glycolysis
Pyruvate transported into the mitchondrion
Undergoes decarboxylation to produce acetyl-CoA
Reduction is irreversible, requires NAD+, and is catalyzed by pyruvate dehydrosenase complex
Acetyl-CoA proceeds through the tricarboxylic acid (TCA) cycle to produce CO2, NADH, and FADH2
Protons from NADH and FADH2 create the proton gradient required for the production of ATP by the electron transport chain (ETC)
What does glycolysis + TCA cycle + ETC produce?
36 molecules of ATP from oxidation of one molecule of glucose
Under healthy, resting conditions, what % of pyruvate is converted into lactate by LDH?
~10%
Conversion of Pyruvate into Lactate by LDH
Reversible, cytosolic reaction
NADH is oxidized to NAD+
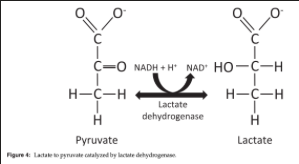
What occurs to continue to supply ATP when oxygen demand exceeds supply?
When oxygen demand exceeds supply, NAD+ stores become depleted, pyruvate and NADH accumulate in the cytosol and LDH activity is upregulated
Lactate formation reduces cytosolic pyruvate and H+ while replenishing NAD+, enabling glycolysis to continue supplying ATP
Once oxygen supply is restored, LDH transforms lactate back into pyruvate which can enter the TCA cycle or be used for gluconeogenesis
What is metabolic acidosis that occurs with hyperlactatemia the result of?
Hydrolysis without concurrent proton consumption by the ETC and subsequent proton accumulation
Lactate as a Strong Anion
Lactate is a strong anion and has an acidifying effects similar to chloride according to Stewart approach
Increase in lactate causes a decrease in the SID
Decreasing the SID results in an increase in [H+] -> acidosis
![<ul><li><p><span>Lactate is a strong anion and has an acidifying effects similar to chloride according to Stewart approach</span></p><ul><li><p><span>Increase in lactate causes a decrease in the SID</span></p></li><li><p>Decreasing the SID results in an increase in [H+] -> acidosis</p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/63cb3aa5-7fcb-4ef1-8119-d71b4633cf63.png)
In the quantitative approach to acid base how does lactate affect SBE?
For each 1 mmol/L increase in lactate, SBE will decrease by 1 unit
Lactate Transport
Transport across cell membranes occurs predominantly via facilitate passive transport by proton-linked monocarboxylate transporters (MCT) and sodium-coupled MCTs
MCT1 and MCT4 most important in mammalian tissues
Lactate transporters also play an essential role in "lactate shuttles," a form of energy currency exchange
Shown to exist in the brain, striated muscle, liver, kidneys, and myocardium
Where does the majority of lactate produced at rest come from?
Skeletal muscle (40-50%), the brain (13%), and adipose tissue (variable)
What % of lactate production in the blood are RBCs responsible for?
80%
What % of lactate production in the blood are leukocytes (predominately neutrophils) responsible for?
13%
What % of lactate production in the blood are platelets responsible for?
7%
What are the most important lactate consuming tissues?
Liver (20-30%), the renal cortex (20%), and the myocardium (5-15%)
Hepatic lactate uptake is a saturable process
Where in the kidney is lactate reabsorbed?
Proximal convoluted tubule
What causes Type A hyperlactatemia?
Due to insufficient oxygen supply
What causes relative Type A Hyperlactatemia?
Due to insufficient oxygen supply from increased oxygen demand
What causes Absolute Type A Hyperlactatemia?
Due to insufficient oxygen supply from inadequate oxygen delivery
What causes Type B Hyperlactatemia?
In the face of apparently adequate oxygen availability
What causes Type B1 Hyperlactatemia?
Associated with underlying disease
What causes Type B2 Hyperlactatemia?
Associated with drugs or toxins
What causes Type B3 Hyperlactatemia?
Resulting from congenital errors in metabolis
Relative Type A Hyperlactatemia
Can occur due to exercise, seizure activity, shivering, trembling, and struggling
Exercise induced hyperlactatemia is highly variable
In healthy animals, lactate concentrations fall rapidly following cessation of muscle activity, with an estimated half life of 20-60 minutes
Seizure-induced hyperlactatemia results primarily from vigorous muscle activity and is associated with a similar half-life
A persistent increase in lactate after cessation of seizure activity is concerning
Absolute Type A Hyperlactatemia Due to Shock
Likely the most common cause of pathologic hyperlactatemia in veterinary ECC
Shock is associated with inadequate oxygen delivery to the tissues, leading to impaired mitochondrial respiration and increased anaerobic metabolism
Onset of hyperlactatemia relative to oxygen delivery is similar in hypovolemic, cardiogenic, and obstructive shock, but occurs earlier in maldistributive shock due to impaired oxygen extraction from mitochondrial and microcirculatory dysfunction
Hyperlactatemia of shock is unlikely to be solely due to impaired oxygen delivery leading to increased anaerobic metabolism
Several causes of Type B hyperlactatemia are believed to be important contributors to shock-associated hyperlactatemia