IB SL Chemistry - Structure 2.4
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
30 Terms
What is Materials Science?
The interdisciplinary field studying the structure, properties, processing, and performance of materials, including the development of new materials. It draws from chemistry, physics, and engineering.
What is the concept of the Bonding Continuum?
The idea that the main bonding types (ionic, covalent, metallic) are not absolute categories but rather extremes on a continuous spectrum. Many substances exhibit characteristics intermediate between these pure types. This spectrum allows for a better understanding of material properties and behaviors based on the degree of bonding present.
What is the van Arkel-Ketelaar Triangle (Bonding Triangle)?
A triangular diagram used to represent the bonding continuum. The three vertices represent pure ionic, covalent, and metallic bonding. A substance's position within the triangle indicates the relative contribution of each bonding type.
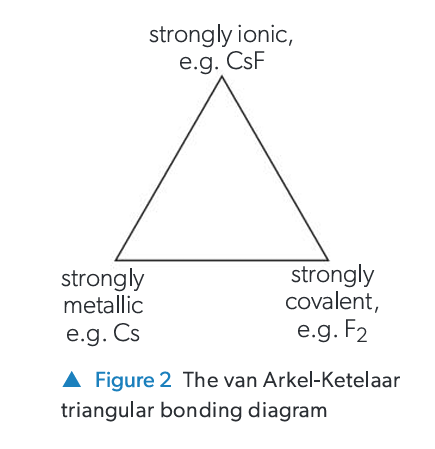
How is a substance's position determined in the Bonding Triangle?
By calculating two parameters based on the electronegativity (χ) of the bonded elements:
Electronegativity difference (Δχ) - related to ionic/covalent character.
Mean electronegativity (x̄) - related to metallic/covalent character.
Define Brittleness. Which bonding types often lead to brittle materials?
Brittleness is the tendency of a material to fracture or break when subjected to stress, with little deformation. Ionic compounds and covalent network structures are often brittle. This occurs because the rigid structure of ionic bonds or covalent networks does not allow for flexibility, leading to fractures under applied forces.
Define Elasticity. Give examples.
The ability of a material to deform under stress but return to its original shape when the stress is removed. Examples: Metal springs (metallic bonding), rubber bands (polymer chain uncoiling).
Define Plasticity. Give an example.
The ability of a material to undergo permanent deformation under stress (it keeps its new shape). Example: Modeling clay.
What is Corrosion? Give a common example.
The gradual destruction of a material, usually a metal, by chemical reaction with its environment. Example: Rusting of iron (oxidation in presence of O₂ and H₂O).
What is an Alloy?
A mixture consisting mainly of a metal, combined with one or more other elements (which can be metals or non-metals).
Why are alloys considered mixtures rather than compounds?
Their composition (ratio of components) can vary within a range, and the components generally retain their individual properties.
How do the properties of alloys typically compare to their constituent pure metals?
Alloys often have enhanced properties like increased hardness, strength, and corrosion resistance, or modified melting points. They usually retain metallic conductivity and luster but are often less malleable/ductile.
Explain why alloys are generally harder/stronger than pure metals.
The different-sized atoms of the added element(s) disrupt the regular arrangement of the metal lattice, making it more difficult for layers of atoms to slide past one another under force.
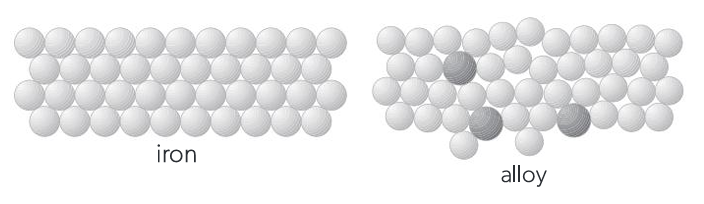
What is Steel? What property is significantly enhanced compared to pure iron?
Steel is an alloy of iron and carbon. It is significantly harder and stronger than pure iron. This enhanced strength and hardness are primarily due to the presence of carbon, which modifies the crystalline structure of iron.
What is Stainless Steel and why is it resistant to rusting?
An iron alloy containing at least 11% chromium. The chromium reacts with oxygen to form a thin, continuous, protective layer of chromium oxide (Cr2O33) on the surface, preventing further corrosion (rusting).
What are Bronze and Brass?
Bronze: Alloy of copper and tin (hard, corrosion-resistant).
Brass: Alloy of copper and zinc (malleable, good acoustic/antimicrobial properties).
Define Polymer and Monomer.
Monomer: A small molecule that acts as the basic repeating structural unit.
Polymer: A large molecule (macromolecule) formed by linking many monomer units together via covalent bonds.
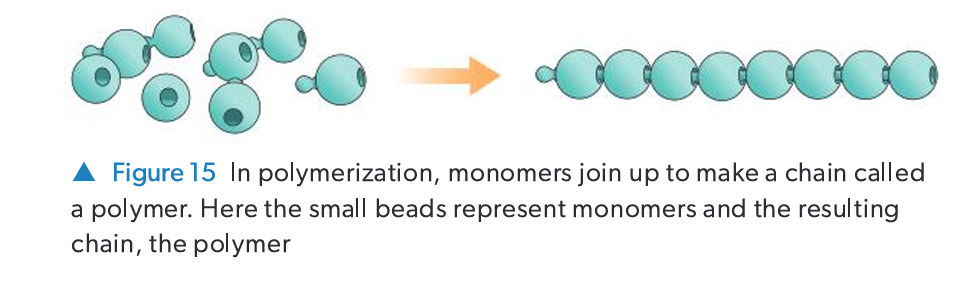
What is Polymerization? Name the two main types.
The process of joining monomers to form a polymer. The two main types are:
Addition Polymerization
Condensation Polymerization
What is a Repeating Unit in a polymer?
The specific arrangement of atoms, derived from the monomer, that repeats itself along the polymer chain. Polymer structures are often shown by drawing the repeating unit in brackets with a subscript 'n'.
Differentiate between Natural, Synthetic, and Semi-synthetic Polymers.
Natural: Produced by living organisms (e.g., cellulose, starch, DNA, rubber).
Synthetic: Man-made (e.g., polyethene, PVC, nylon, Kevlar).
Semi-synthetic: Made from modified natural polymers (e.g., cellophane from cellulose).
Describe the general properties of polymers.
Usually low density, poor electrical/thermal conductors. Relatively high melting points (for molecular substances) due to cumulative IMFs between long chains and entanglement. Strength varies greatly depending on structure and IMFs.
Describe Addition Polymerization. What type of monomer is required?
Monomers add to each other without the loss of any atoms. The polymer contains all atoms of the original monomers. Requires monomers with a carbon-carbon double bond (C=C), which breaks to form linking single bonds.
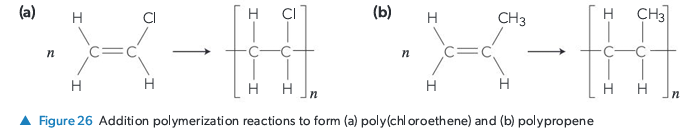
Describe Condensation Polymerization.
Monomers (with suitable functional groups) join together by eliminating a small molecule (like H2O) for each link formed between them.
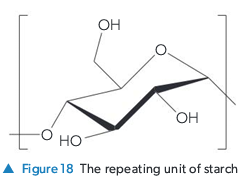
Compare the structures and properties of Cellulose and Starch.
Both are glucose polymers.
Cellulose: Linear chains, extensive H-bonding between chains leads to high strength, insolubility (plant structure).
Starch: Branched chains, less efficient packing, fewer H-bonds, weaker, digestible (energy storage).
What are Plastics?
A common term for synthetic polymers, often composed mainly of carbon and hydrogen. They are typically chemically unreactive and durable. Most can be molded into different shapes and are used in a wide range of applications, from packaging to construction.
What is Crosslinking in polymers? How does it affect properties?
Formation of covalent bonds between different polymer chains. Crosslinking increases strength, rigidity, and reduces solubility and biodegradability.
Define Biodegradation. Why are many synthetic plastics non-biodegradable?
The breakdown of materials by the action of microorganisms. Many plastics resist biodegradation because their stable covalent bonds (e.g., C-C, C-H) lack reactive sites for microbial enzymes to attack.
What are Bioplastics?
Plastics derived from renewable biological sources, such as corn starch or vegetable oils, rather than petroleum. They may or may not be biodegradable.
What are Microplastics? How do they form?
Tiny plastic particles (< 5 mm). They form from the physical breakdown and fragmentation of larger plastic items, not from chemical biodegradation.
What are Smart Materials? Give an example related to polymers.
Materials designed to respond predictably to changes in their external environment (e.g., pH, temperature). Example: pH-responsive hydrogels that swell or contract based on acidity/basicity.
What are Hydrogels?
Crosslinked hydrophilic polymer networks that can absorb and retain large amounts of water, forming a gel. They are used in various applications such as wound healing, drug delivery, and as contact lenses.