Module 2.4: Charges and Buffers
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
What affects the overall net charge on a molecule?
pH
How do you calculate the overall charge on a molecule as a function of pH?
Identify all ionizable groups on the molecule & their charge when protonated (qHA) and deprotonated (qA-).
Use the known pKa of each group to determine the fraction protonated (fHA) and deprotonated (fH-) at the required pH.
Calculate the overall charge by summing the contribution of each group using the following formula. qTotal=∑fHAqHA + fA-qA-
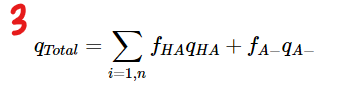
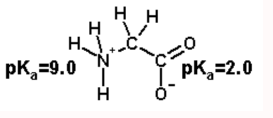
Calculate the net charge on the amino acid glycine at pH 2.0. Glycine at pH=7.0
Look back at the walkthrough with a green star for help
Answer: +0.5
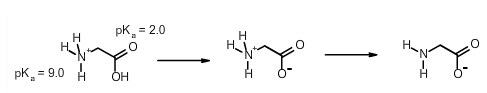
Select the best estimate the net charge on the amino acid glycine at pH 3.0:
+1.0
+0.1
+0.0
-0.1
-1.0
Answer: +0.1
(look back at the charges and buffers notes for the work on how to do this)
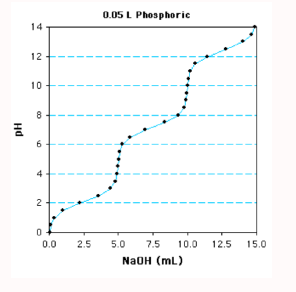
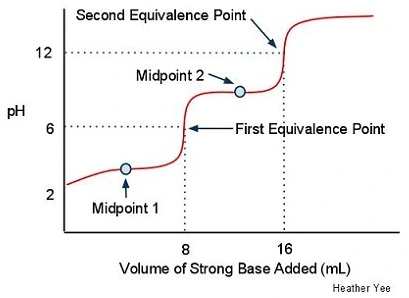
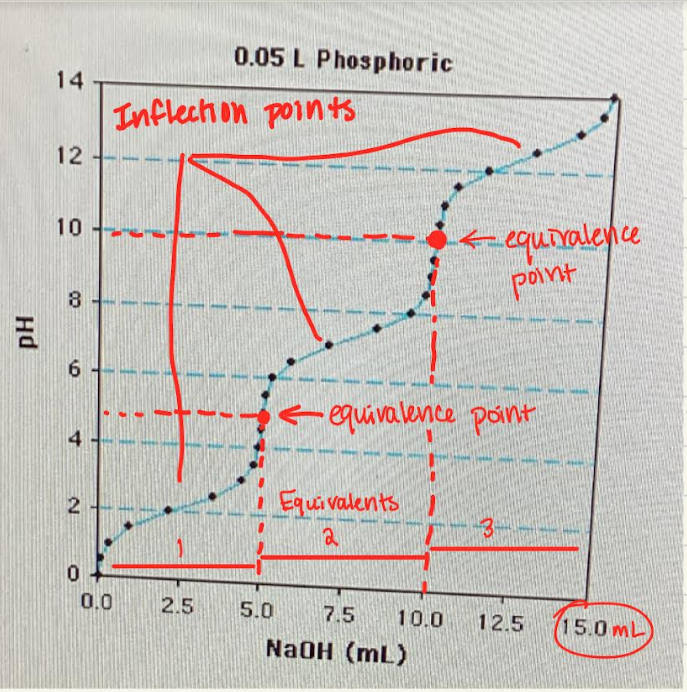
Label the equivalents, equivalence points, and inflection points
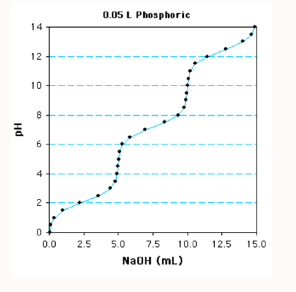
The following is a titration curve for what type of acid?
triprotic acid
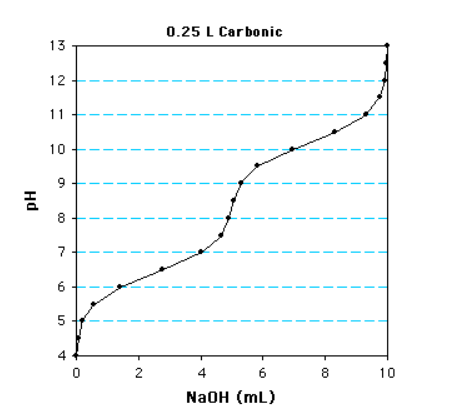
The following is a titration curve for what type of acid?
diprotic acid
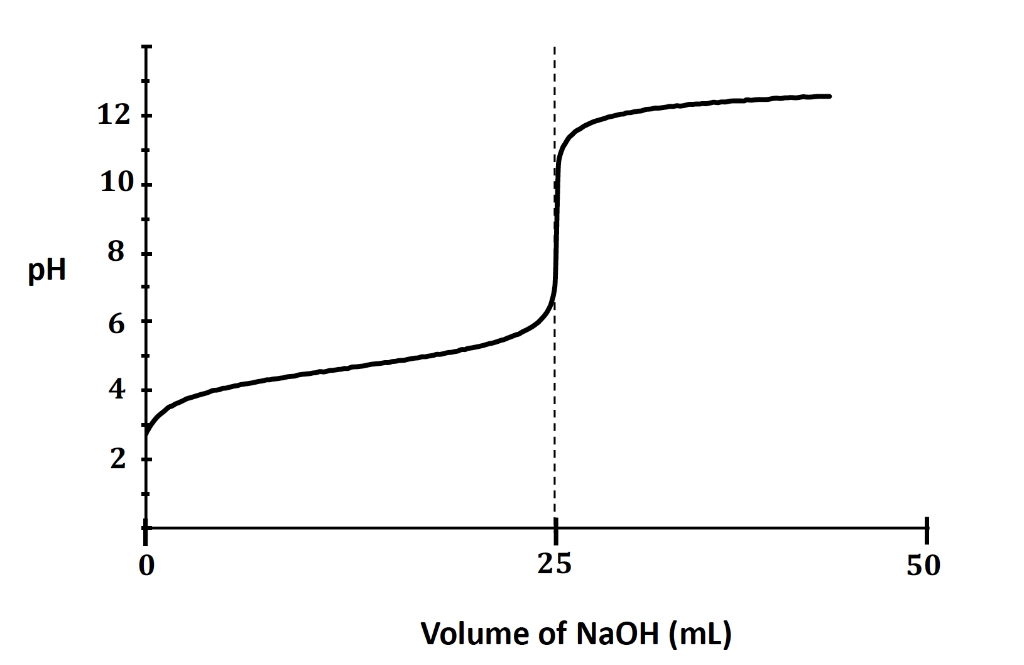
The following is a titration curve for what type of acid?
monoprotic acid
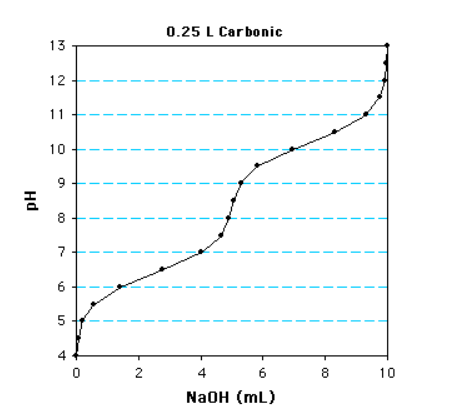
The first pka value is? What is the pH?
6.5 and 6.5 (because pka=pH since the concentration of the acid equals the conjugate base)
What is the concentration?
10 mL is required to fully deprotonate the acid (aka total volume)
0.010 L x 1 mole/L = 0.010 moles
0.010 moles / 2 (diprotic) = 0.005 moles
0.005 moles / 0.25 L = 0.02
Answer: 20mM
What is a buffer? Why is it important?
A mix of a weak acid + conjugate base.
Importance: A buffer resists changes in pH by absorbing or releasing protons
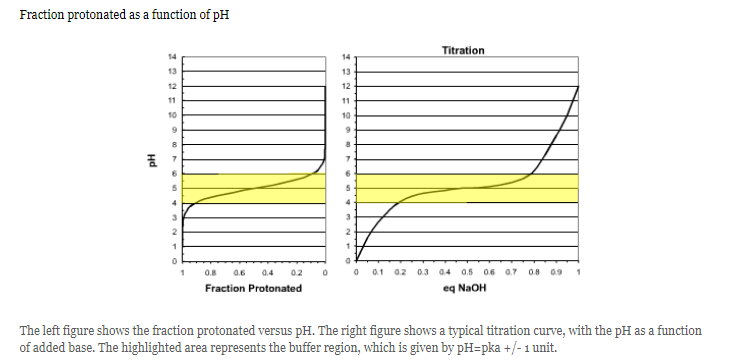
Interpret this graph
At the beginning of the titration, the pH is much lower than the pka, so the acid is fully protonated (meaning it has a lower pH = more acidic)
Then the curve approaches the midpoint when the pH=pka along with the yellow buffer range
The acid becomes fully deprotonated as it reaches the higher pH
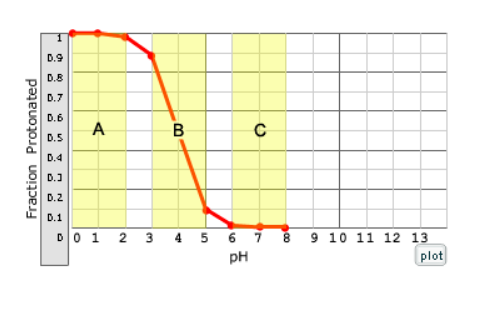
Which region of the curve on the right corresponds to a solution that will not resist pH changes due to the addition of a strong acid?
A, this region cannot resist changes because the weak acid is fully protonated in region A and can't accept additional protons.
What is the buffering range?
This is the pH range over which the pH changes slowly when acid or base is added. The buffer range is considered to be pH values that are one unit below to one unit above the pKa
What is the buffer capacity?
This is the total amount of base (or acid) that can be added to a buffer solution and still have the pH with the buffer range. It depends on the concentration of the weak acid and the starting pH.
What is the formula for the Henderson Hasselbalch equation?
pH = pka + log [A-]/[HA]
![<p>pH = pka + log [A-]/[HA]</p>](https://knowt-user-attachments.s3.amazonaws.com/8589953c-682a-4bd3-83c5-13b5bfa62baf.png)
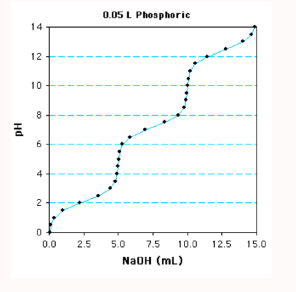
Determine the concentration of the acid
Find the number of moles: A total of 15 mL of 1 M NaOH is required to fully deprotonated (0.015 L x 1 mole/L = 0.015 moles)
Divide by # of ionizations to obtain moles of weak acid: (0.015 L / 3 = 0.005 moles) Since it is a triprotic acid
Divide the volume of solution to obtain molarity of weak acid: (0.005 moles / 0.05 L = 0.1 M or 100 mM)
If [A-] > [HA] then…
pH > pka
If [HA] > [A-] then…
pH < pka
We measure a pH of 5.0 for a solution of acetic acid (pka 4.8). What is the ratio of base to acid? What is the fraction of acetic acid?
What if we added 0.2 mol of acetic acid to the 1 L of water, then it reached the current equilibrium. What is the concentration of acetic acid now?
pH=pka + log ([A] / [HA])
5.0=4.8 + log ([A] / [HA])
0.2= + log ([A] / [HA])
100.2 = ([A] / [HA]) is the ratio answer
fHA= 1/1+R
fHA= 1/1+100.2 = 0.39
39% of the molecule is acetic acid
0.2 mol / L x 0.39 = 0.078 M of the acid form
0.2 mol / L x 0.61 = 0.122 M of the base form
Ka values, or acidity constants, must be measured by direct experiment, usually with a ____
pH titration
What does a titration measure?
A strong base (NaOH) is added to a solution of weak acid and the pH is measured as the amounts of NaOH is added
As the base is added, it removes the protons from the acid (which increases pH by removing the free protons from water)
What are the equivalents?
the x-axis scale for titrations is given in moles of base/moles of acid.
Therefore, it varies from 0 to 1 for an acid that releases one proton (monoprotic), from 0 to 2 for a diprotic acid, 0 to 3 for a triprotic acid.
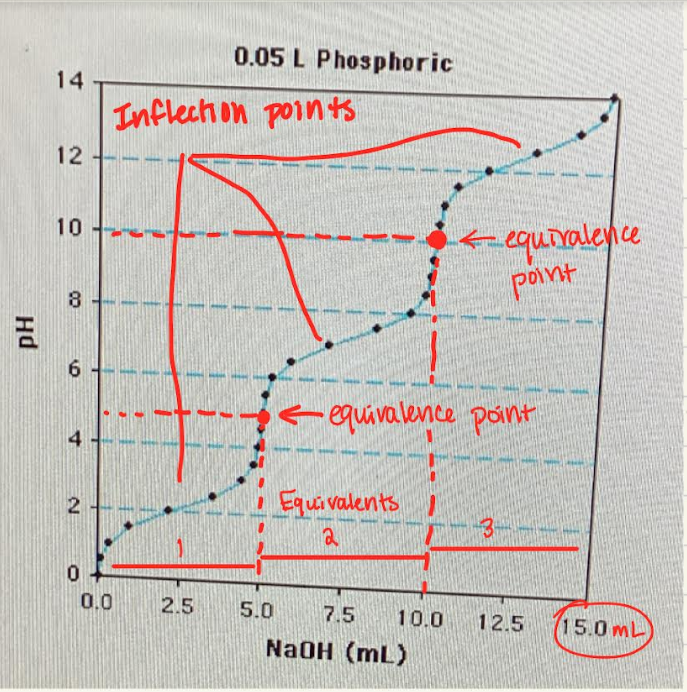
What are the equivalence points?
Marks when the base has completely removed all the protons (aka deprotonation) from the weak acid
At this point, you can find the concentration of the acid
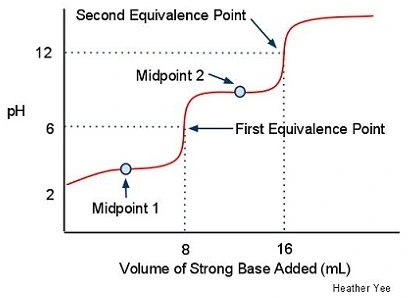
What are inflection points?
The midpoints where pH=pka