Chapter 13 (Incomplete - dilutions, limiting reagents and excess reagents to be calculated)
1/66
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
67 Terms
Separation of compounds
In a reaction pathway, intermediate compounds and by-products are also formed
Separation of the compounds enables us to get the desired product from the mixture, disregarding the by-products
Intermediate compound
A substance that is identified between the change from a reactant to a product
Distillation
A way of separating two or more liquids based on their boiling point
The difference in boiling point between the mixtures must be at least 50 degrees for effective separation
How it works
The vessel with the mixture is placed at the bottom - a tube connects it to a round-bottom vessel at the top
The liquid with a lower boiling point travels up the tube and is collected at the top vessel (distillate)
Is condensed back into a liquid by a water-cooled condenser
Visual explanation (Insert here)
Distillate
The vapor which is condensed and returned as back into a liquid
Fractional Distillation
A process of distillation used when the boiling points of the reactants are very similar, and it may be harder to seperate/distinguish them
The distillate has a higher concentration of species that have a lower boiling point
The original mixture of liquids has a higher concentration of mixtures that have a higher boiling point
How it works
Mixtures with a higher boiling poinjt condense in the fractionating column, as the heat is not high enough for them to continue as a gas
Vapours with a lower boiling point continue and are seperated as the distillate
Note: Heat gets lower as the height increases
Visual Of Fractionating Column (Insert)
What is it commonly used for
To separate volatile liquids (liquids that easily vaporize at low temperatures)
Anti-bumping granules
Small and irregular shaped stones in fractionating column where condensation of higher boiling point species occurs
Textbook explanation

Ethyl Ethanoate Fractional Distillation
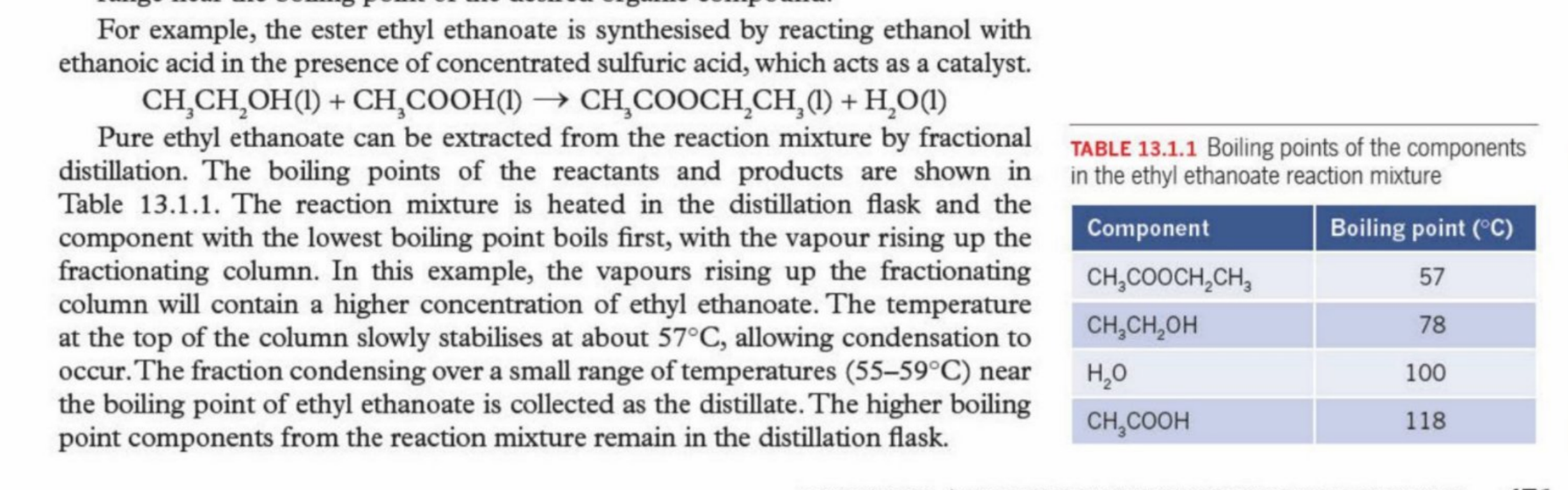
Explanation
Ethyl Ethanoate - Lowest boiling point - hence higher proportion is sent to distillate
Melting Point Determination
A measurement of when the solid first begins melting, to when the solid has completely melted
Utilises a melting point range
Melting point rangwe
The temperature difference from when the solid first shows signs of melting to when it has completely finished melting
If pure - 0.5-2 degrees (the range) - is called as narrow
If impure - the temperature difference is more than that - broadens the melting point range
Why impurities have a broader melting point range
Impurities are when multiple organic structures combine - is impure
Impurities introduce different particles into the lattice.
These particles:
Distort the lattice
Create regions with weaker intermolecular forces
Are unevenly distributed throughout the solid
Some parts are pure - melt quicker
Some parts are impure - melt slower
As a rnage - it has a broader melting point raneg
Why impurities have a lower melting point
If pure - lattice is structured and very compact - has strong bonding due to its crystalline nature
If Impure - branching and an irregular structure contributes to decreasing the overall melting poitn
Why pure structures have sharp/narrow melting point range
A pure solid has particles arranged in a uniform, repeating lattice.
This means all particles require about the same amount of energy to overcome the intermolecular forces → sharp melting.
If melting point of compound is higher
It is not the desired product
If the melting point of the compound is lower
It is either impure or not the desired product
Mixed Melting Point Determination
When a pure sample is mixed with an unknown sample
If this new mixture has the same melting point range and melting point of the known substance, then it is pure
If not it is impure
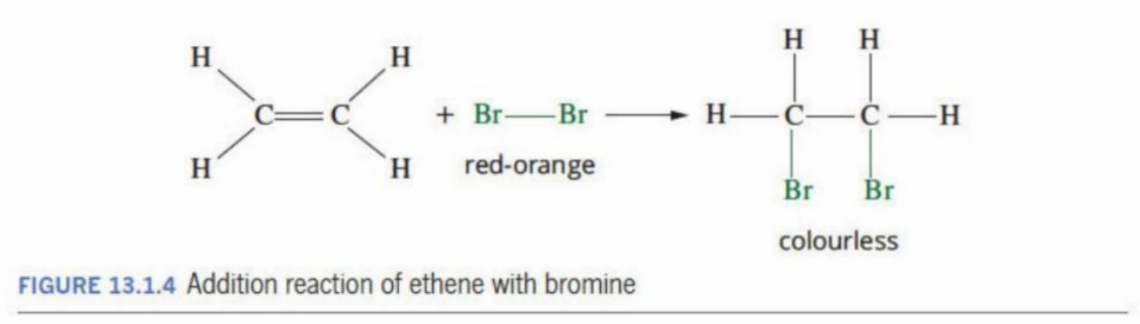
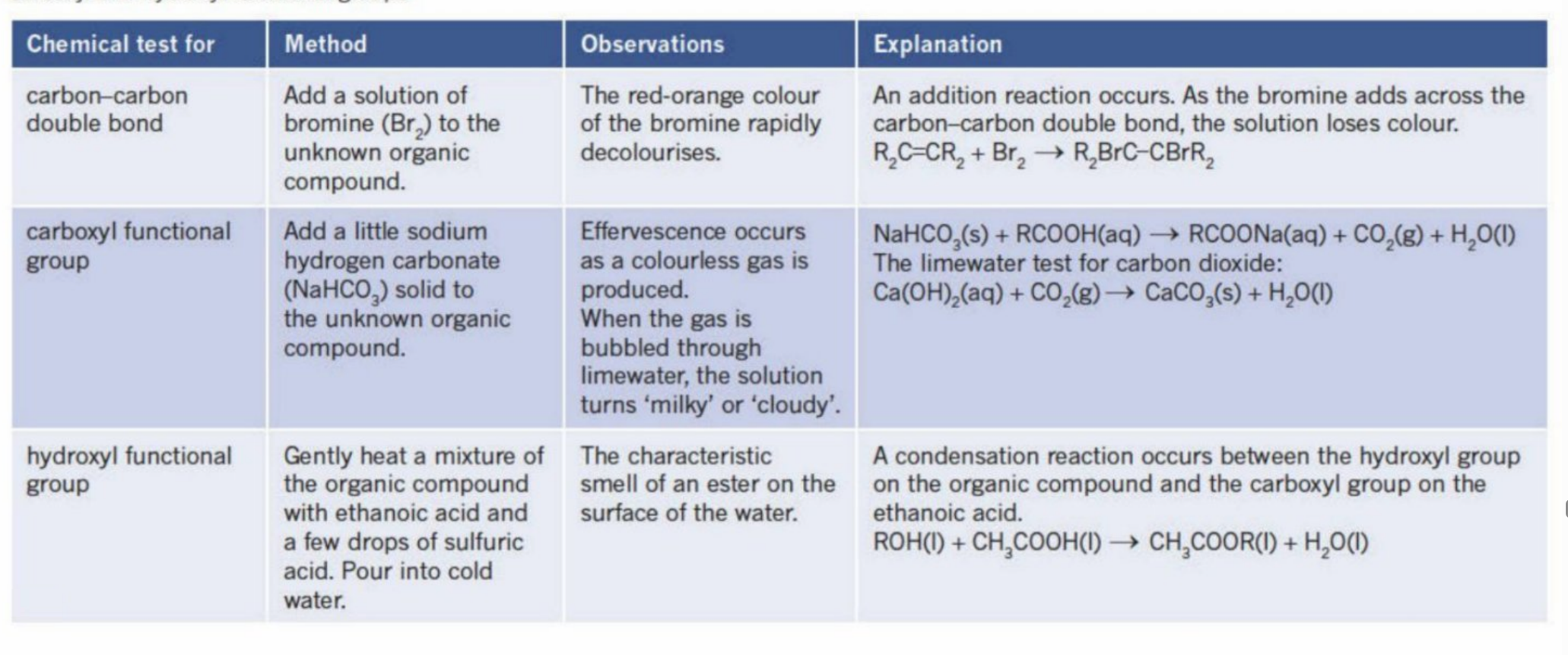
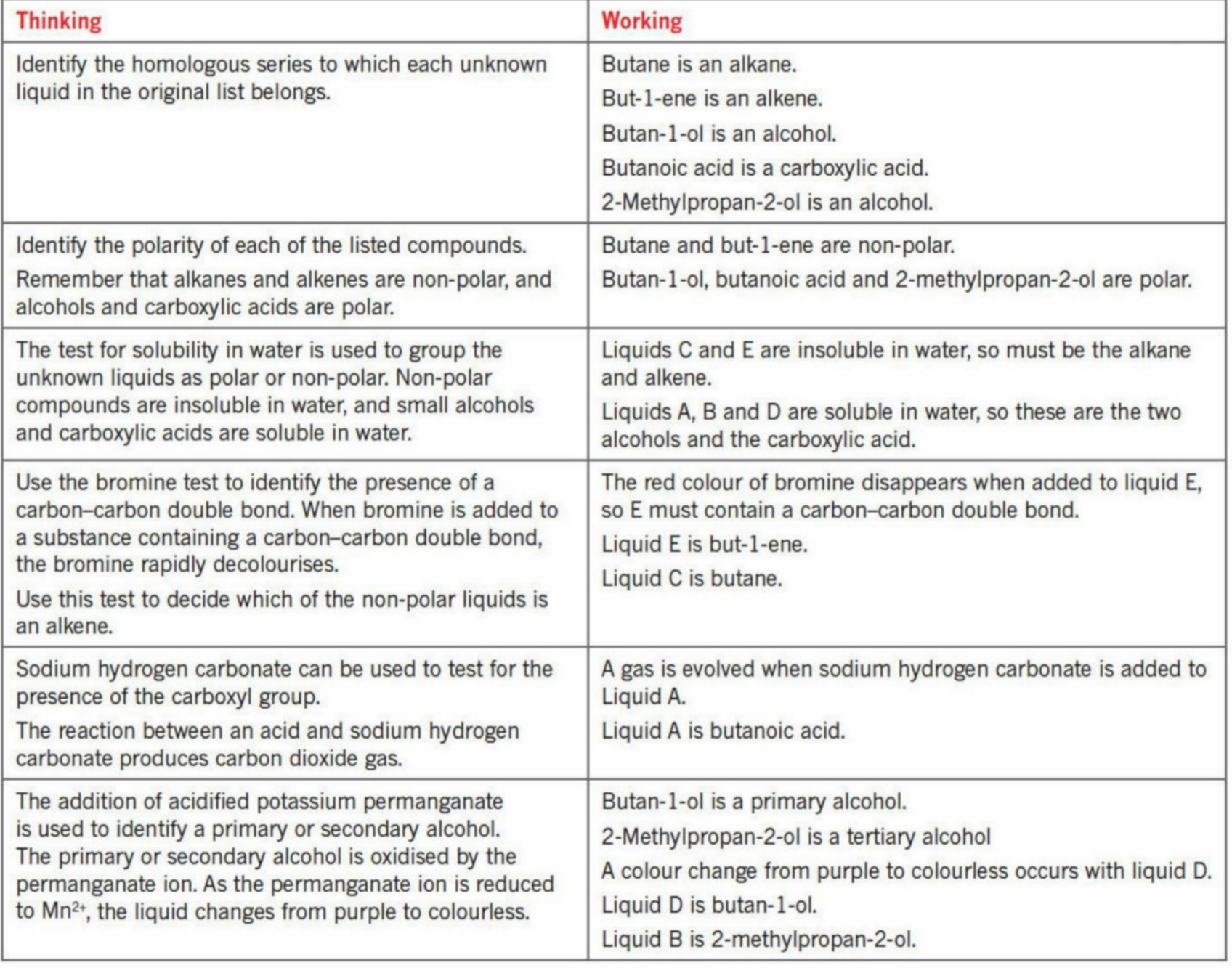
Identifying Prescence of functional groups (C=C bond)
Adding a liquid such as bromine (red or red-orange in color) to the alkene
As it is unsaturated, the alkene undergoes an addition reaction and takes in the Bromine atoms - rapidly decolourises the solution
The color of the solution thus turns colorless
Visual Representation
Testing Prescence for hydroxyl group
Is mixed with a carboxylic acid + sulfuric acid (as a catlyst)
If an ester is formed - then there is hydroxyl group

Testing if it’s a tertiary alcohol or primary and secondary alcohols (Review Chapter 11)
Primary and secondary alcohols oxidize easily but tertiary alcohols do not
So when adding acidified potassium dichromate or acidified potassium permanganate - if it changes color - then primary or secondary alcohol
If not - tertiary
If manganese
The permanganate turns into Mn+2 which is colourless
Testing for Prescence of carboxyl group
As the carboxyl group is acidic, when it reacts with a carbonate (acid and base reaction), it produces CO2 gas
Acid + carbonate (or bicarbonate) —→ Salt+ Water + CO2
This can be confirmed by doing the limewater test (tube connecting the system to the limewater - if cloudy/milky, then CO2 was produced)
Visual representation (Insert here)
Summary for testing all functional groups
Example (Must do)
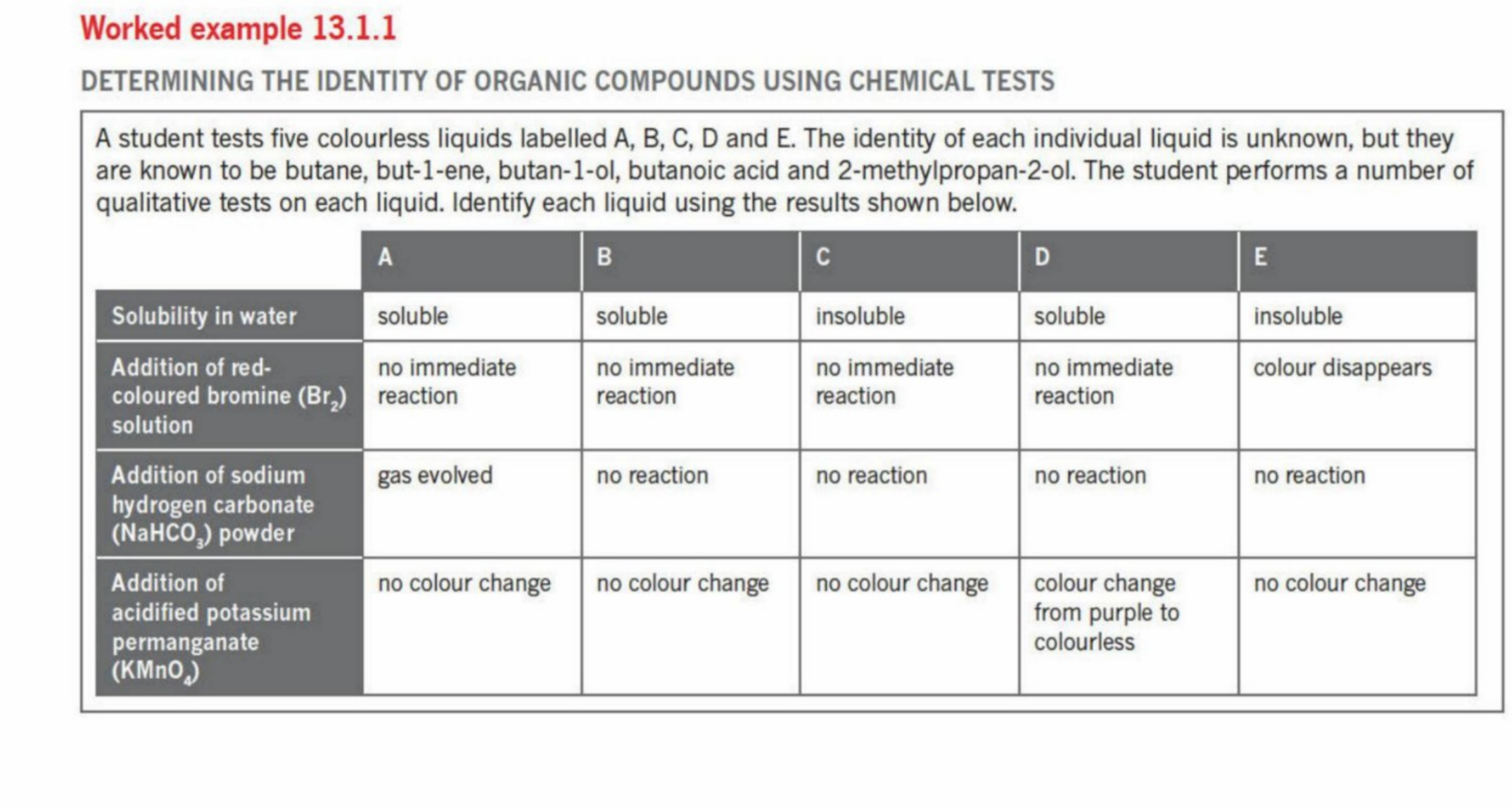
Solutions
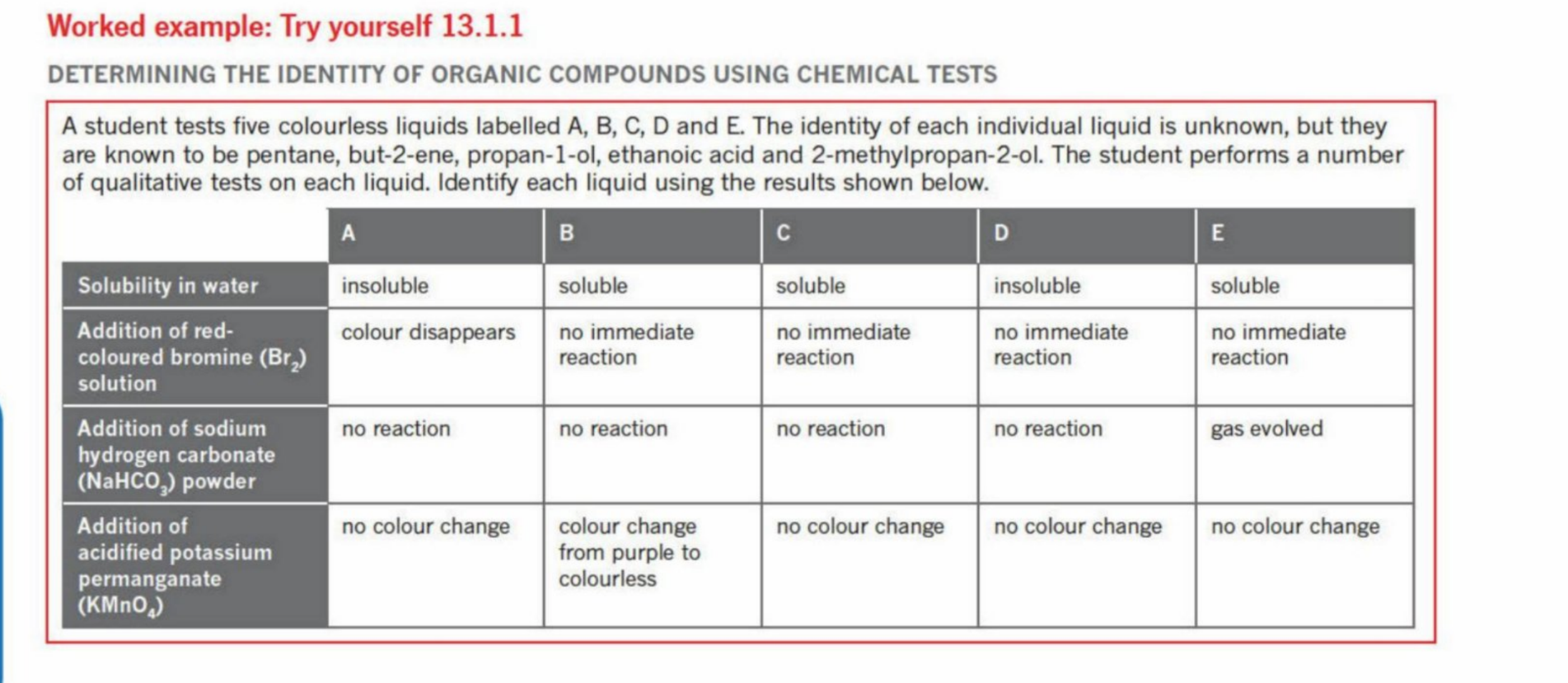
Example 2
Are tertiary alcohols soluble
Yes
if short chains - very soluble because OH bond is polar (proportion is equally matched)
if longer chain - not much - the polar bond is outweighed by the chain’s non-polar characteristic
Example 2 Answers
A - But-2-ene
B - Propan-1-ol
E - Ethanoic Acid
D - Pentane
C - 2-Methylpropan-2-ol
Instead of Bromine, why is Iodine more preferable to use in checking for C=C bonds?
More stable
Less dangerous - Aqueous bromine releases harmful fumes of bromine - creates respiratory hazard
How is Iodine prepared to make it a suitable alternative to Bromine (unclear)
Making it standardised using a sodium thiosulfate solution
How Iodine number is calculated
As one mole of I2 reacts with one mol of C=C bonds, the nymber of moles of I2 = the no. of moles of C=C bonds in the fatty acid molecule
Must find how much I2 reacts with 100 g of that molecule
Example
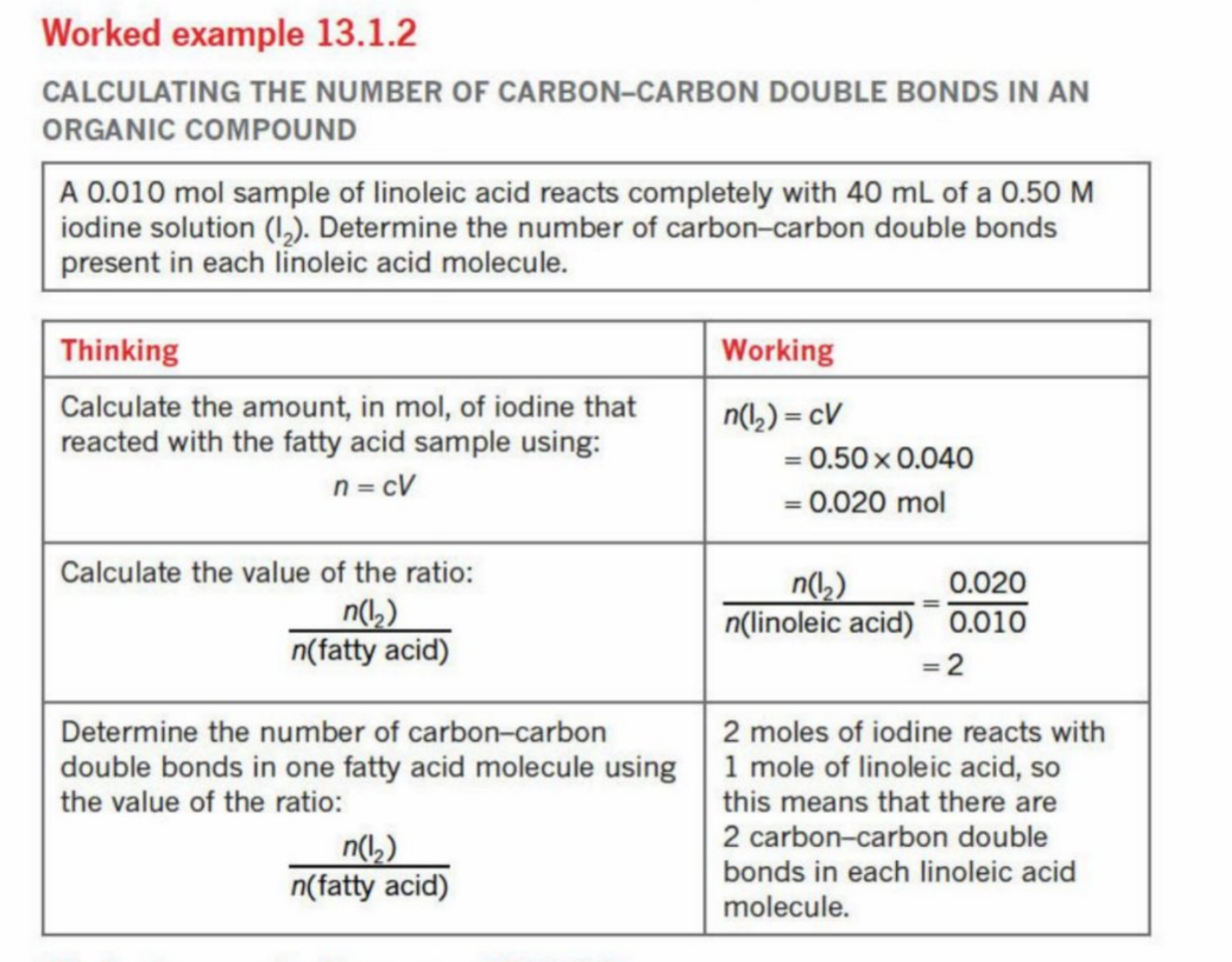

Worked Example (Insert Solution)
Standard Solution
A solution of accurately known concentration
Primary Standrad
A very pure substance, such that its moles can be calculated accurately from its mass
Conditions of a primary standard

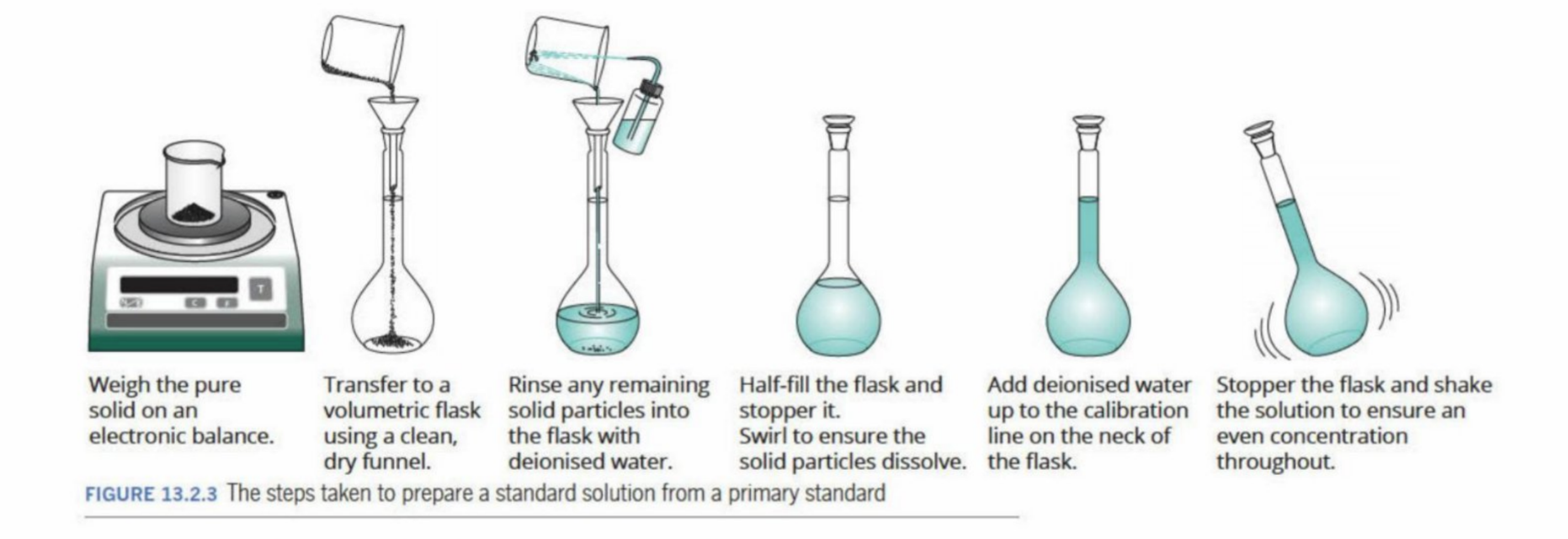
How standard solutions are prepared
Dissolving accurately measured mass of primary standard in water to make an accurately measured volume of solution
Using titration with another standard solution to find its concentration
If a a standard solution is to be made from something that is not a primary standard (already pure)
Primary standard → can make a solution directly (accurate from weighing).
Impure/unstable chemical → must be titrated first → then you get a standardised solution.
Steps In Tiration
Equivalence Point (Key wording)
When the reactants have when the amount of titrant added is exactly enough to completely react with the analyte.
The reactants are in stoichiometric proportion as indicated in the equation
Endpoint
Observable change in the titration’s colour - signals that the equivalence point is reached - shows that reaction is very close to or is completed
Steps of titration

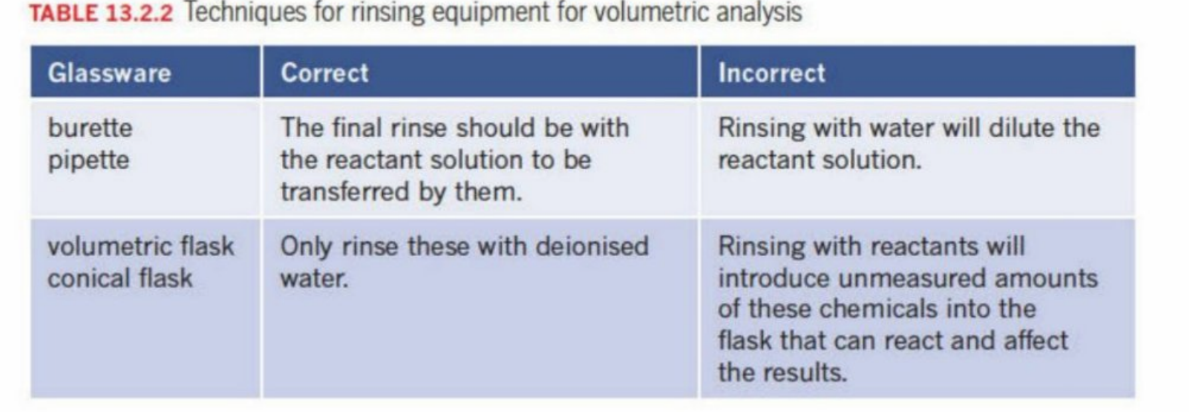
How To Rinse Glassware
Steps Of Volumetric Analysis
How far should concordant titres be away from each other
by 0.1ml (maximum gap)
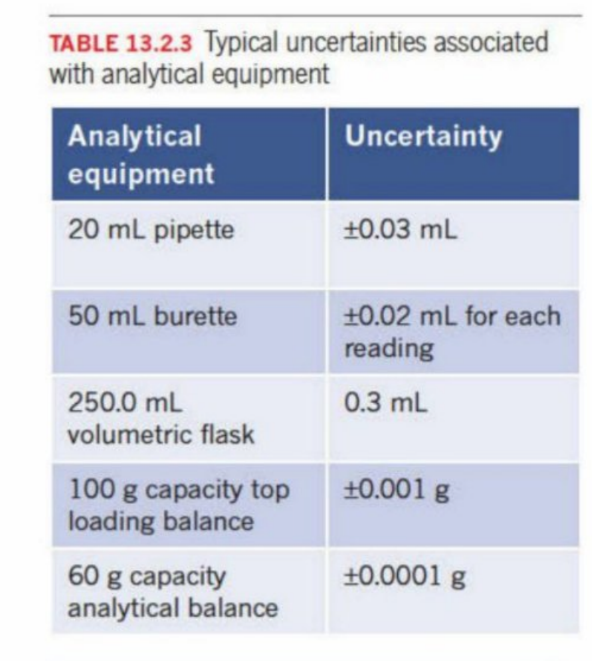
Errors In Volumetric Analysis (Refer to chapter 1 errors)
Depends on the calibration and the accuracy of the materials used
Quantitative analysis - aim to make as accurate as possible
typical errors—→
Redox titration
Reaction of an oxidising agent with a reducing agent (one solution is pipetted and another one is dispensed into the flask)
Adding indicator to redox titrations
Some molecules (e.g. those having MnO4-) don’t need an indicator - it will change color by itself
For other molecules in redox, an indicator (e.g. starch solution) is needed to represent the equivalence point
Purpose
To check the composition of the substances
To check when they reach equivalence point
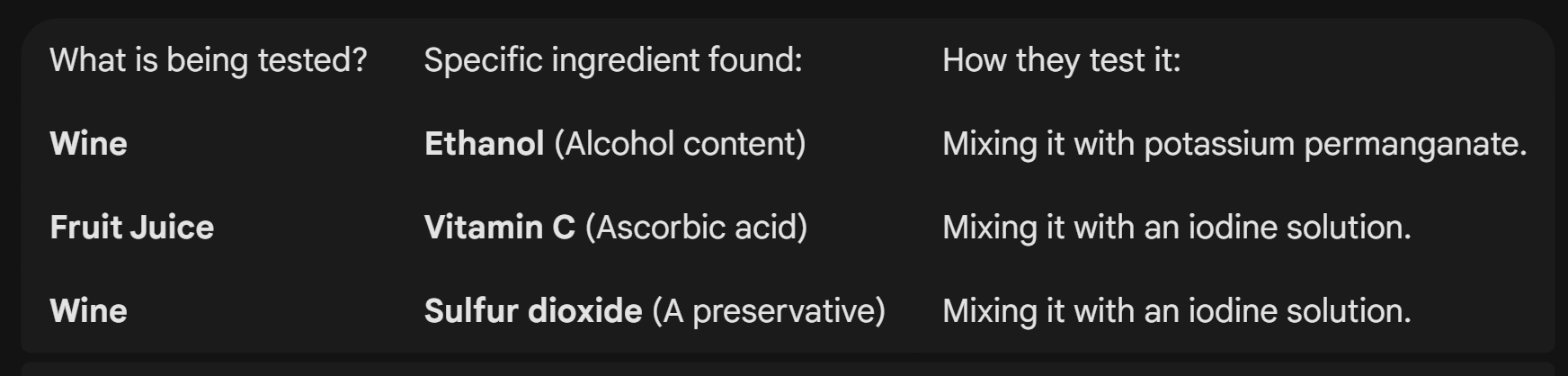
Examples
(e.g. by mixing iodine in fruit juice, we can test for the presence of Vitamin C)
Malic Acid
An acid that gives many fruits their “sour” taste
Is a dicarboxylic acid and an alpha-hydroxy acid (AHA).
It plays a vital role in the Krebs cycle (or Citric Acid Cycle), which is the process your body uses to turn food into energy (ATP).
Alcohols undergoing oxidation
Are weak reducing agents (can’t oxidise as much)
They are already partially oxidized with the C-O bond; thus are weaker reducing agents
When oxidized, the OH group turns into a COOH group (carboxylic acid)
Why Alcohols are weak reducing agents
Ethanol is partially oxidised
Strong C–H and C–O bonds
Electron density pulled towards oxygen
This makes oxidation:
kinetically slow
less favourable
Criteria for oxidising weak reducing agents such as ethanol
Must have very strong oxidising agent (can pull electrons towards itself)
Must have very harsh conditions:
Heat (reflux)
Acidic conditions
Time)
Steps on titration (unclear)
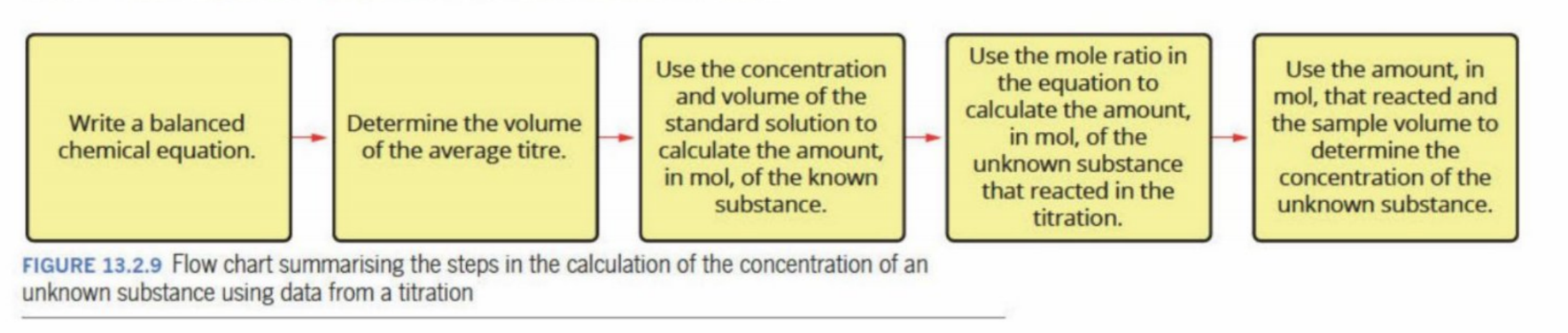
Example (has longer working out)
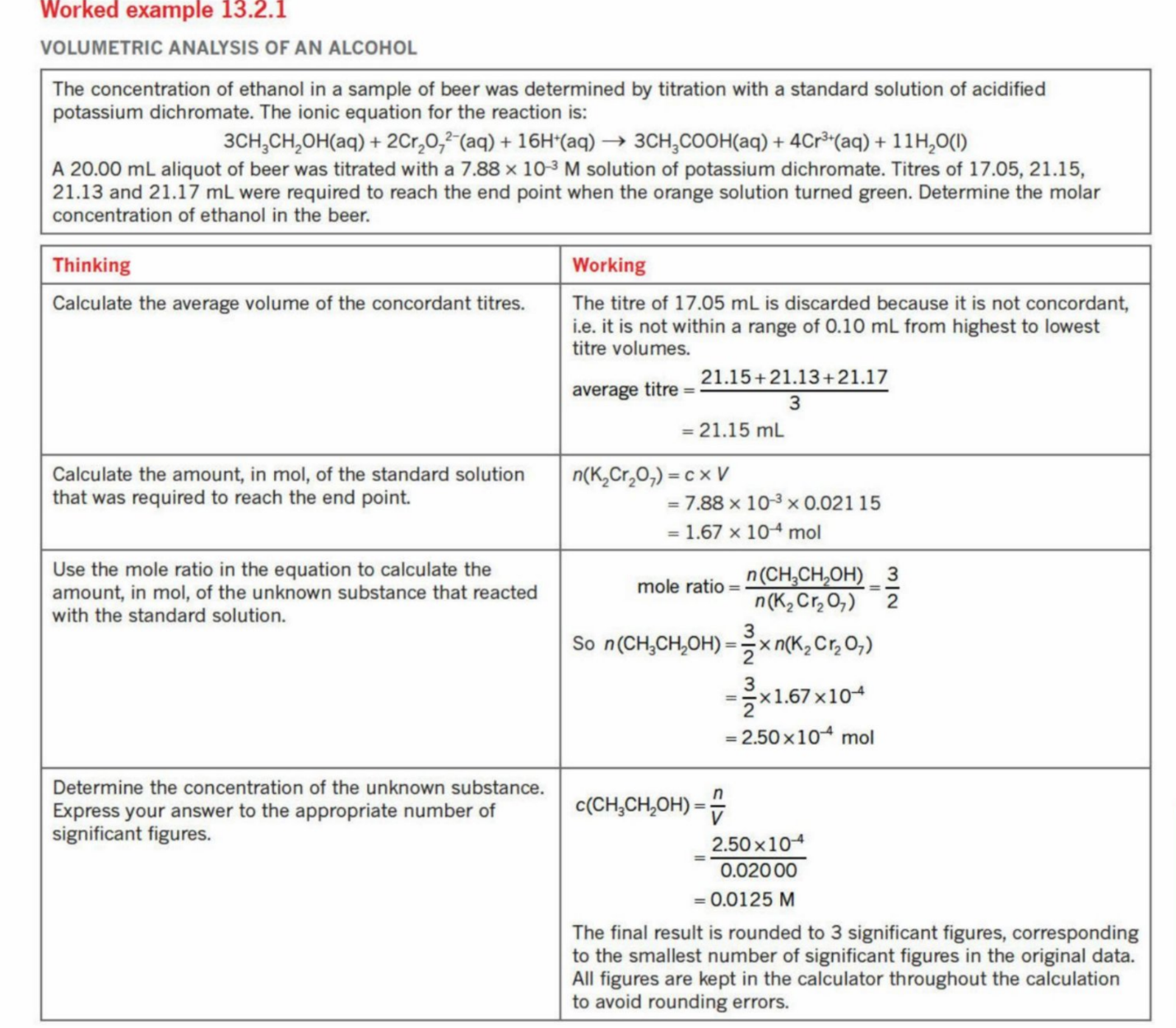

Worked example (shorter working) - Insert solution here
Criteria of indicators for redox titrations
Must be weak oxidizing or reducing agents (so that they don’t react too soon, causing a color change that doesn’t reflect the equivalence point accurately)
Must change color drastically (big change) in their reduced or oxidized form - to indicate equivalence point
ChatGPT explanation