Molecular orbital theoryZ
0.0(0)
Card Sorting
1/19
There's no tags or description
Looks like no tags are added yet.
Last updated 5:21 PM on 5/12/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
1
New cards

D
2
New cards

A-CO
3
New cards

A-three electrons in bonding orbitals and 2 in antibonding orbitals
4
New cards

C-B2 will have unpaired electrons
5
New cards

C-the electron comes from an antibonding MO in oxygen but from a bonding MO in nitrogen
6
New cards
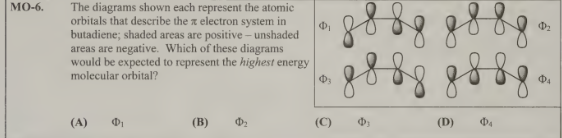
A
7
New cards

A- 1s a(1)1sb(2)+1sb(1) 1sa(2)
8
New cards

A-the born-oppenheimer approximation
9
New cards

A
10
New cards

A-pi g
11
New cards

B-Be2
12
New cards
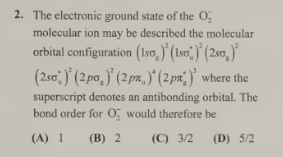
C-3/2
13
New cards
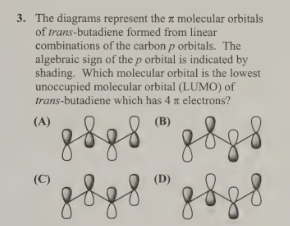
D- Lumo is every other and Homo is every 2
14
New cards
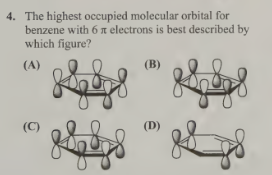
B
15
New cards
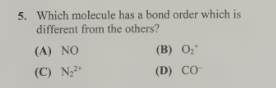
C-N2²+
16
New cards

D-6
17
New cards

A
18
New cards

C-both have cylindrical symmetry about the bond axis
19
New cards

A-greater
20
New cards
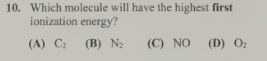
B-N2