HL Chemistry Unit 3
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
49 Terms
Activation Energy
Minimum of input energy required for a chemical reaction to occur (get reactants to transition state when bonds are broken and able to form products)
Higher activation energy → slower reaction
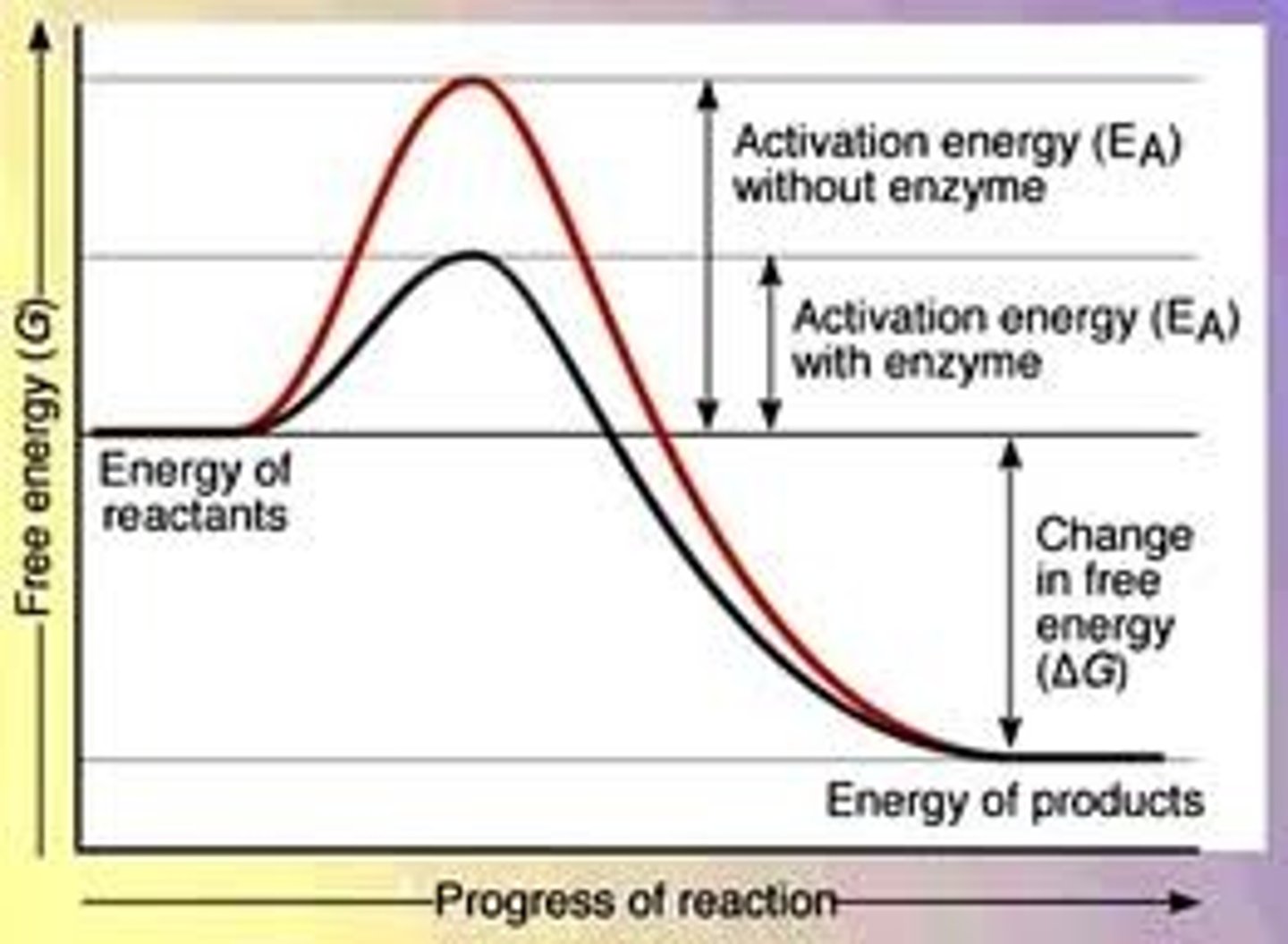
Catalyst
Speeds up a chemical reaction without being consumed during the reaction
Increases the rate of reaction by decreasing the activation energy
Why are d-block elements good catalysts?
Incomplete d-orbitals and ability to have multiple oxidation states
- Weak bonds with reactants, supplies exterior electrons from 3d and 4s orbitals
- Able to take back electrons after the reaction so that the transition metal remains unchanged
Large surface area absorbs reactants so that they are in closer contact, making the reaction faster
Why are dark bottles used to store hydrogen peroxide? What is the catalyst for this reaction?
Dark bottles protect exterior light, which can provide activation energy, causing a reaction for hydrogen peroxide to decompose
MnO2 catalyst
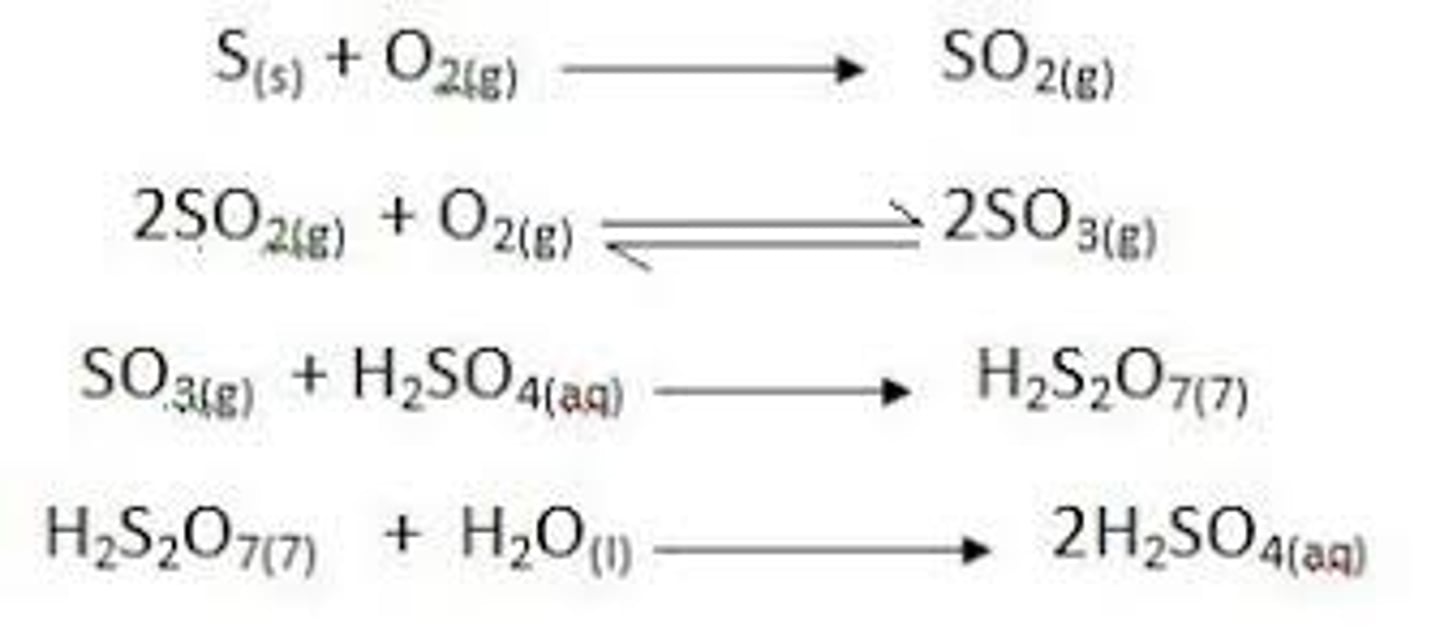
What is the catalyst of the contact process?
Extracts sulfur, can convert sulfur dioxide to sulfur trioxide, and converts sulfur trioxide into concentrated sulfuric acid
Uses of sulfuric acid: manufacture explosives, acids, dyes, glue, car batteries
Vanadium(V) oxide catalyst
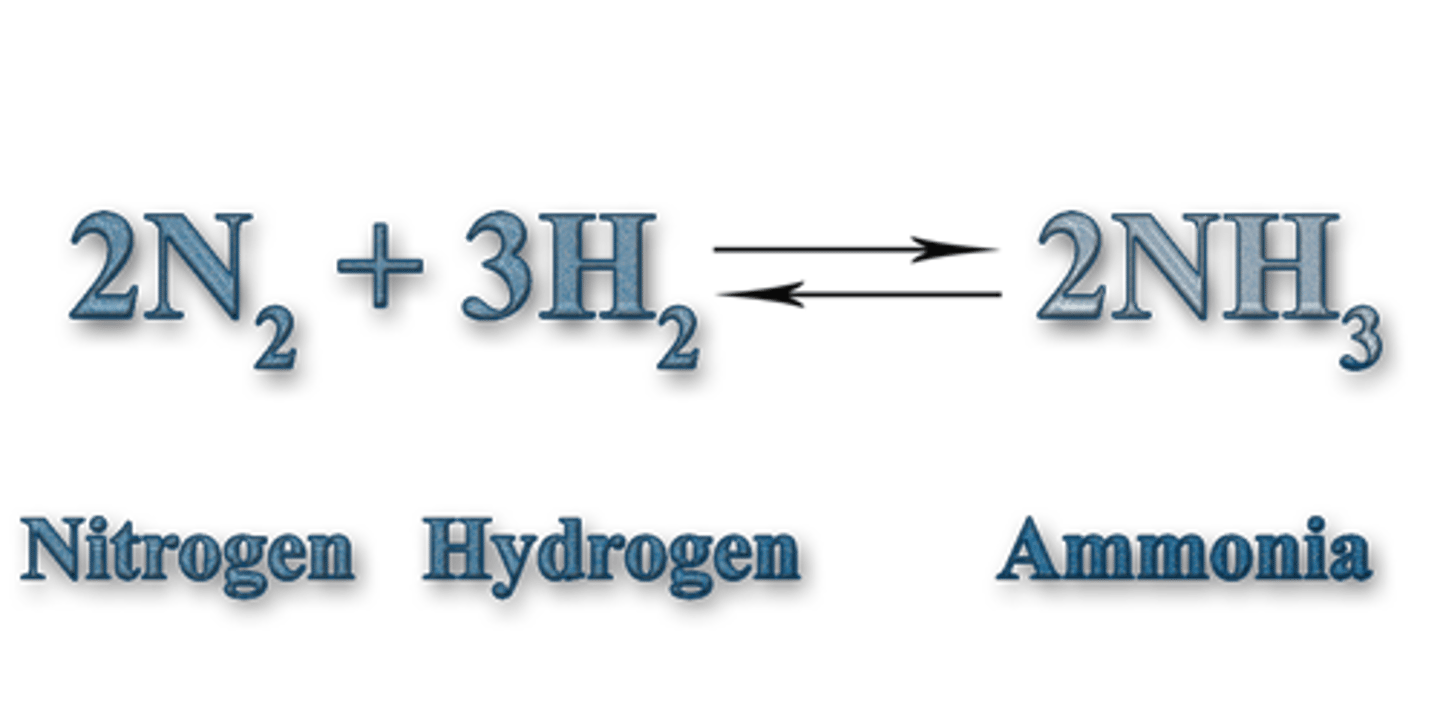
What is the catalyst of the Haber Process?
Converts hydrogen and nitrogen to ammonia, reversible reaction
Uses: manufacture plastics, explosives, textiles, pesticides, dyes, refrigerant gas
Iron catalyst
What is the catalyst in a Heme Group?
Ring-shaped iron-containing molecular component of hemoglobin, found mostly in lungs and tissues
Iron attaches with hemoglobin molecule or myoglobin, needed to bind and transport oxygen throughout body, helps in respiration, detoxification of drugs
What is the catalyst for the conversion of an alkene to an alkane?
Alkane: Carbon and hydrogen compound where each hydrogen is bonded to a carbon and carbon-carbon single bonds hold the atoms together
Alkene: Hydrocarbon with at least one carbon to carbon double bond, more reactive than alkane because of double bond
Nickel is used to convert the alkenes to alkanes when making trans and saturated fats, and margarine

What does Vitamin B-12 (cobalamin) do?
Nerve cell function, red blood cell formation, DNA synthesis
Cobalt in the center, 4 coordination bonds with nitrogen, connected by C-CH3 methylene link and C-H
Acts as a catalyst for methylation reactions and isomerization reactions
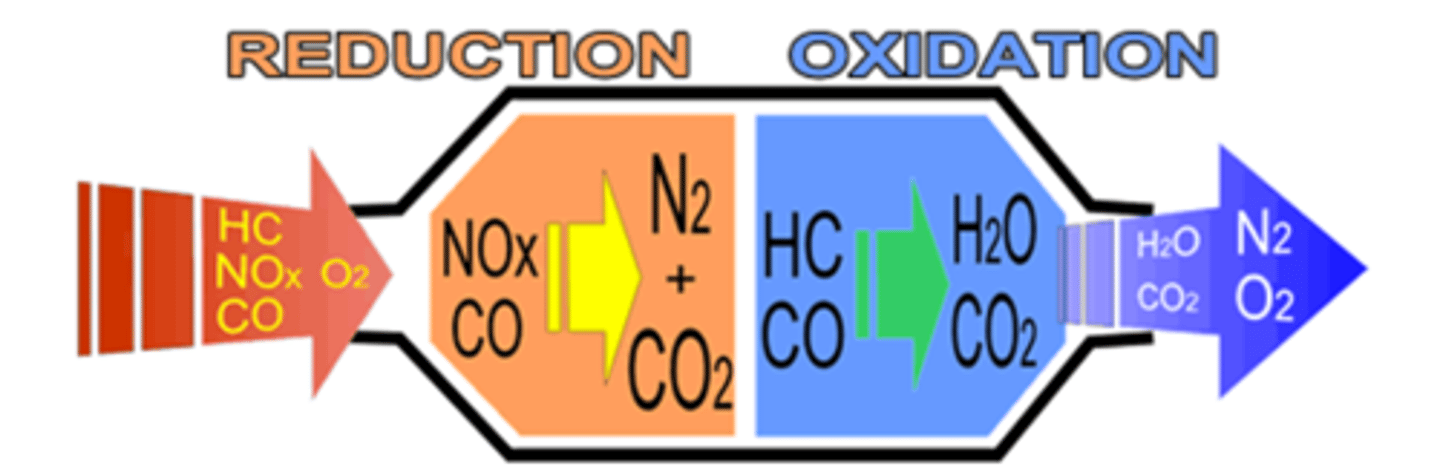
What is the catalyst for the Catalytic Converter?
Converts most hydrocarbons, carbon monoxide, and nitrogen oxides into carbon dioxide, nitrogen, and water vapor
Makes pollutants from car less harmful by chemically converting them
3 way: Converts nitrogen oxides to elemental nitrogen and oxygen, carbon monoxide to carbon dioxide, hydrocarbons to carbon dioxide and water
2 way: oxidation of carbon monoxide and oxidation of hydrocarbons to carbon dioxide and water
Pt/Pd catalyst
VSEPR Theory
Valence Shell Electron Pair Repulsion theory
Pure Covalent Bond
Electrons shared evenly between atoms, even charge distribution
Non-polar Covalent Molecule
Dipoles cancel out
Polar Covalent Bond
Uneven distribution of electrons
Polar Covalent Molecule
Dipoles reinforce in one direction
Ion-dipole interaction
Negatively charged ions attract slightly positive atoms in molecule, positive charged ions attract slightly negative → Ionic compounds in polar solvents
Intermolecular Forces of Attraction
Intermolecular (inter → between) force (attractive force between molecules that holds solids and liquid together. tends to be weak between gaseous molecules)
Boiling water ≠ breaking bonds in molecule ⇒ breaking force/bond between molecules, still same substance
Collective referred to as "Van der Waals Forces"
Aggregates
Groups of molecules held together by intermolecular forces to create liquid and solids (gas forces are too weak)
London Dispersion Forces
Caused by instantaneous, temporary, momentary, random dipoles → random motion of electrons (clouds of probability → uneven distribution of charge)
Only intermolecular force between nonpolar molecules and in nonpolar aggregates
All molecules experience London Dispersion Forces
Not all molecules are participating in attraction at the same time → attraction is weak
Reinforced by induced dipoles (temporary dipole on one molecules forces a temporary dipole on an adjacent molecule)
Factors of strength of LDF
Larger molecules → more mass + electrons → stronger temporary dipoles → more force, stronger LDF
Larger surface area → more points of contact → stronger LDF
Linear > spherical molecules
Dipole-Dipole Interactions
Between polar molecules
Slightly negative end of one molecule is attracted to slightly positive end of an adjacent molecule
Result of permanent dipoles on polar molecules
Ordered fashion → molecules arrange in crystal structure
Hydrogen Bonds
Stronger D-D interaction but can happen (rarely) with instantaneous dipoles
Oxygen dissolves in hydrogen due to hydrogen bond
Only polar molecules → pair of lone electrons on F,O, N (very electronegative) and hydrogen (very electropositive) on different molecule with F,O, N
Hydrogen is attracted to lone pair of different F, O, N molecule
1/10 strength of covalent bond
Larger aggregate → more lone pairs available on F, O, N for hydrogens
Creates 3d crystal structure affect ⇒ why water is more dense frozen than liquid
Small molecule usually gases at room temperature, but strong hydrogen bonds allows it (water) to stay liquid
Intramolecular Force
Forces of attraction within molecules
Covalent bonds
Ionic bond: Chemical bond → forms ionic compounds → does not form molecules → not inter/intramolecular
Ionic Solid Bonding model
Anions and cations
Crystal lattice structure
- Simple cubic
- Body centered cubic
Bond strength
Larger ions → weaker ionic bonds (pack less tightly together than smaller ions )
Atom size increases → bond length increases → decrease in bond strength
Larger magnitude of charge → stronger ionic bond
Smaller interionic distances (more tightly packed) → more stable
Ionic Solid Properties
Brittle, not malleable
Hard → strong uniform forces
High melting/boiling point
Bonds hard to break
Electrical Conductivity as a liquid + aqueous
Free-moving ions, ions have dissociated with each other
Metallic Solid Bonding Model
Electrostatic attraction between cations and an electron cloud
Bond strength
- Radius of ion
- Charge of ion
Metallic Solid Properties
Luster
- Energy from photons absorbed, excites electrons, falls down to emit light
Malleable/ductile
- Non-directional bonds: Cations can slide around without needing to remove any strong bonds
Electrical conductivity as a solid and liquid
- Free-moving and delocalized electrons
- No aqueous, metals do not dissolve in water
Thermal conductivity, transfers heat easily
High melting/boiling point
- Strong metallic bond
Hard → Strong bond
Sonority →Ringing sound when struck
Network solids bonding model
Repeated structure of covalent bonds
3D
- Repeated vespr shapes
- All covalent
- Hard to break
2D
- Covalent bond within layers
- LDF between layers
1D
- Chain are strong covalent bonds
- Weak LDF within chains
Network Solids Properties
High melting/boiling point due to strong covalent bonds
Hardness very high due to the rigid 3D structures
Brittle --> Breaking bonds is the only way to move them
Not conductive except for graphene
- Lack free-moving electrons/charges
Heat conductivity is variable (ex. diamond is very conductive, while silicon dioxide is not)
Allotropes of Carbon
Carbon atoms bonded covalently in a continuous network
- Pure covalent bonds
Crystalline structure
Graphite, graphene, diamond, fullerene, C60 (buckminsterfullerene)
Graphite
Made of layers of graphene
Has delocalized electrons between layers (LDF)
Black but has luster
Opaque
Very soft
'Greasy' feeling
High melting point → strong covalent bonds
Soft → Weak van der waal forces
Electrically conductive
Thermally conductive
Graphene
2D graphite
Single layer of carbon atoms bound in a hexagonal honeycomb lattice
Formed through sp2 hybridization, creating a trigonal planar with 120° angles
Delocalized electron movement through p orbitals in the π bonded network
Lightweight
Transparent
High elasticity and flexibility
Very hard (for a 2D material)
Very thin
Good conductivity
Thermally conductive
Diamond
Atoms arranged in fixed structures, no individual molecules
Overlap of sp3 hybridized carbon atomic orbitals in infinite 3D structure
Graphite can be converted into diamond with high pressure + heat to rearrange bonds
Hardest naturally occurring substance (10 on Mohs Hardness Scale)
Used in cutting tools (e.g. diamond bit drills)
Refractive index of 2.42
High density of 3.50-3.53 g/cm3
Brittle
Varies widely in colour (colourless to yellow and pinks)
Good electrical insulator and good heat conductor
Fullerene
Hexagons and pentagons that form a spherical molecule, attaching a carbon atom to each vertex
Forms hollow "cage-life" structures
Carbon atoms are present in the sp2 hybridization form, linked by covalent bonds (double or single bonds)
Held together by LDF(not strong)
Slippery and excellent lubricants as weak intermolecular forces allow fullerene to slide past each other
Brittle/Soft due to weak Van der Waals interaction
Dark needle-like crystals
Lower melting point than graphene
Low water solubility
Not conductive
C60 (buckminsterfullerene)
Most common fullerene
Has 60 carbons with 20 hexagons and 12 pentagons
Has 90 covalent bonds between them, 60 single bonds and 30 double bonds
Highly structured and symmetrical
Polar molecular solids bond model
Held together by LDF and dipole dipole
- Same changes repel but isn't as significant as opposite attraction
Organised in lattice structure where oppositely charged ends are closest together
Shape affect strength of the bonds
- Solids has stronger bonds → Regular geometric shapes
Polar molecules solids properties
Soluble in polar solvents
Very soft, not often in solid form
Low melting and boiling point
Still higher than non polar
Low vapor pressure and volatility
Insulators
Non-polar molecular solids bond model
Symmetrical electron distribution + held together by LDF (induced dipoles) and strong covalent bonds within molecule
Stretched out shapes → more surface area → more LDF
Non-polar molecular solids properties
Very soft → weak forces
Low melting/boiling point
Weak forces/induced and instantaneous dipoles
High volatility/vapor pressure
Weaker forces/attraction, require less energy to overcome
Hard to undergo chemical change → Non-polar
Low electrical conductivity/thermal conductivity
No free moving electrons
Flexible/malleable/soft/brittle
Soluble in non-polar solvents
Low melting and boiling points
Hybridization
Combination of two or more orbitals (l) in the same energy level (n)
Ensures that electron pairs are at the same energy to interact in VSEPR models
Has energy intermediate between orbitals it came from + leaves last orbital unhybridized
Transition Metals
All are found in d-block (but not all d-block are transition)
Multiple oxidation states (commonly +2)
Can have magnetic properties
- Unpaired d-electrons (spin in same direction)
Atom of 1+ ions with incomplete d-orbitals
Make good catalysts
Transition metals can make "complex ions" with one or more "ligands"
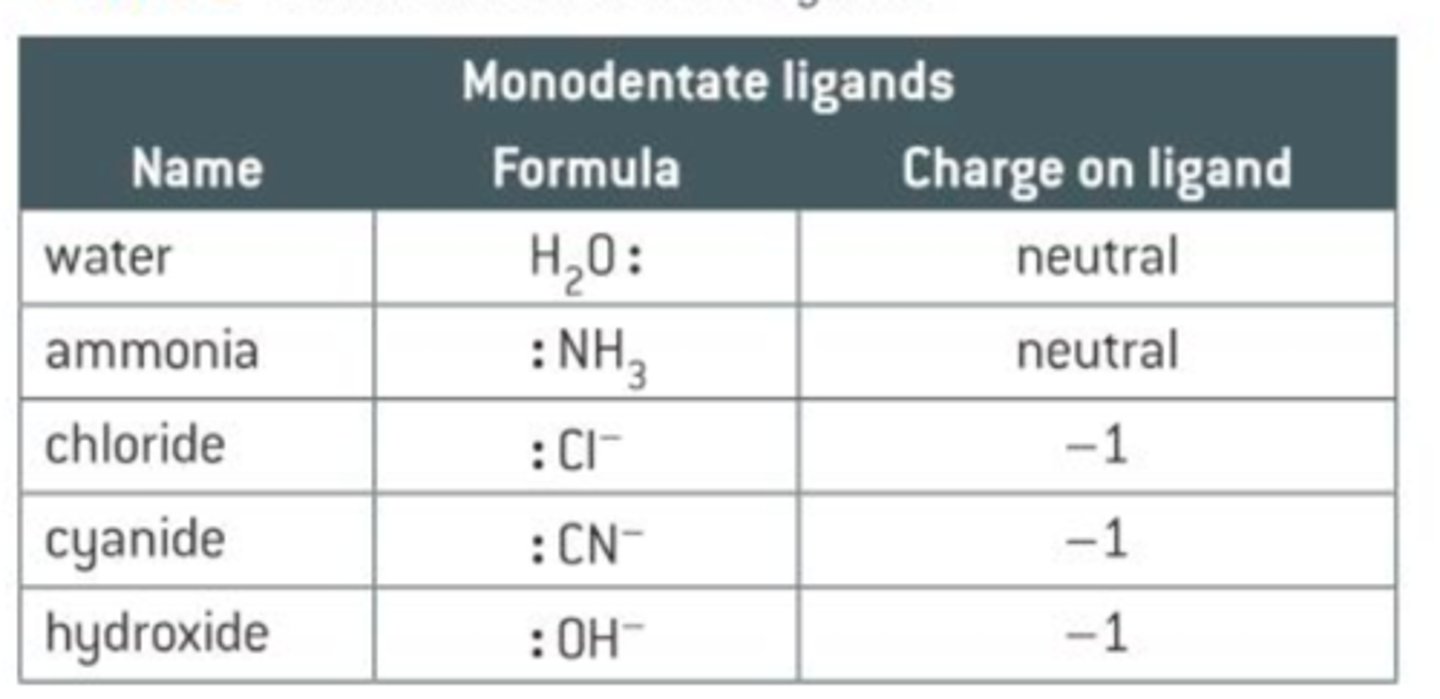
Ligand
Molecule or ion with lone pairs of electrons that can be donated to a coordination bond
mono-, bi-, tri-, tetra-, dentate → how many "teeth" a ligand has → treat as 1 lone pair of electron per ligand
Complex Ion
Metal ion covalently bonded to one or more ligands
Why are transition metal complex ions coloured?
The metal ions will absorb specific colors (color → λ → f → ΔE → Δd) from white light
"Empty spaces" in higher energy split d-orbitals allowing for excitation of lower d orbitals
"Absorption of light is due to electrons in excited/promoted to higher energy levels"
The substance will appear to be coloured the complementary color of the one "removed"/absorbed
Factors that affect the colour of transition metal complex ions
Type of ion: Different metals have different numbers of protons and number of electrons between nucleus and d-orbitals (shielding)
- Changes how attractive metal nucleus is to d-orbitals
Type of ligand: More electronegative ligand will pull on d-orbitals with more forces than a less electronegative ligand
Oxidation state of the metal: Changes number of electrons in the d-orbitals
- Smaller change in colour
Number of ligands (can't change without changing type of ligand): More ligands will pull on d-orbitals with more combined force and vice versa
Orbital Overlap
Orbitals that have been hybridized overlap end-to-end or axially in sigma bonds
Axial Overlap/Head-On/End-to End
Forms sigma bonds
Parallel Overlap
Forms pi bonds
Common ligands
aqua (H2O)
hydroxy (OH-)
amine (NH3)
chloro (Cl-)
cyano (CN-)