Common Polyatomic Ions and Solubility Equilibria
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
Ammonium
NH₄⁺
Acetate
CH₃COO⁻
Permanganate
MnO₄⁻
Nitrate
NO₃⁻
Nitrite
NO₂⁻
Perchlorate
ClO₄⁻
Chlorate
ClO₃⁻
Chlorite
ClO₂⁻
Hypochlorite
ClO⁻
Sulfate
SO₄²⁻
Sulfite
SO₃²⁻
Carbonate
CO₃²⁻
Bicarbonate (Hydrogen carbonate)
HCO₃⁻
Phosphate
PO₄³⁻
Chromate
CrO₄²⁻
Dichromate
Cr₂O₇²⁻
Oxalate
C₂O₄²⁻
Peroxide
O₂²⁻
Thiocyanate
SCN⁻
Thiosulfate
S₂O₃²⁻
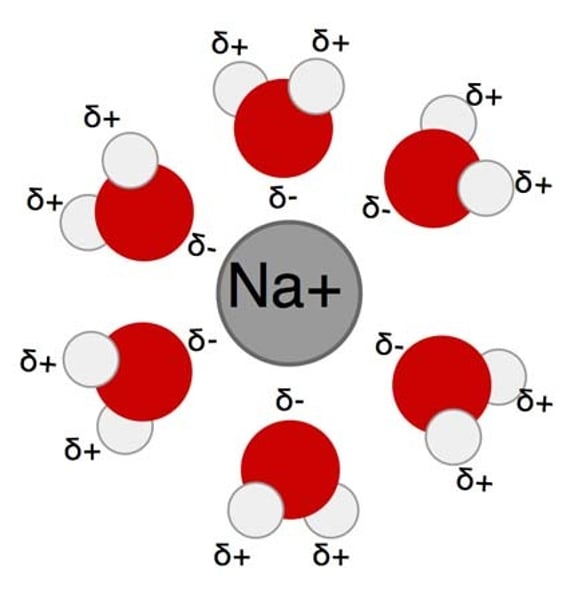
Hydration
Ion broken off and surrounded by polar water molecules
Ksp
Solubility constant (equilibrium constant for soluble compound in soultion)
No
Are pure liquids and pure solids included in equilibrium constant calculations?
Kf
Formation constant for complex ions
Kf*Ksp=Keq
Formula to find equilibrium constant for solubility with complex ion formation