Unit 7 P1 Skills of Chemistry >
7.1 Binary Ionic Compounds
A Binary Ionic Compound is composed of two elements, one is a metal ion (cation) and the other a nonmetal ion (anion).
To write one, we first write both the symbols for the metal and nonmetal, along with their charges. Next, we swap the two charge numbers around and make them the subscript for the other element. You should then simplify the ratios if possible.
Example: Magnesium Phosphide
We know these two elements are a metal and nonmetal because of their location on the periodic table.
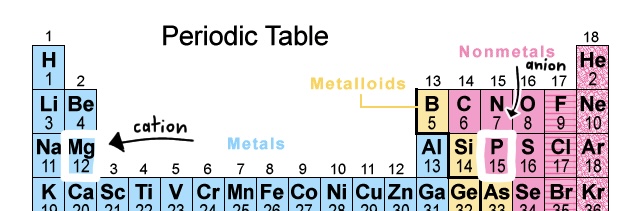
Now we take their symbols, Mg and P, as well as their respective charges
But how do we know their charges?
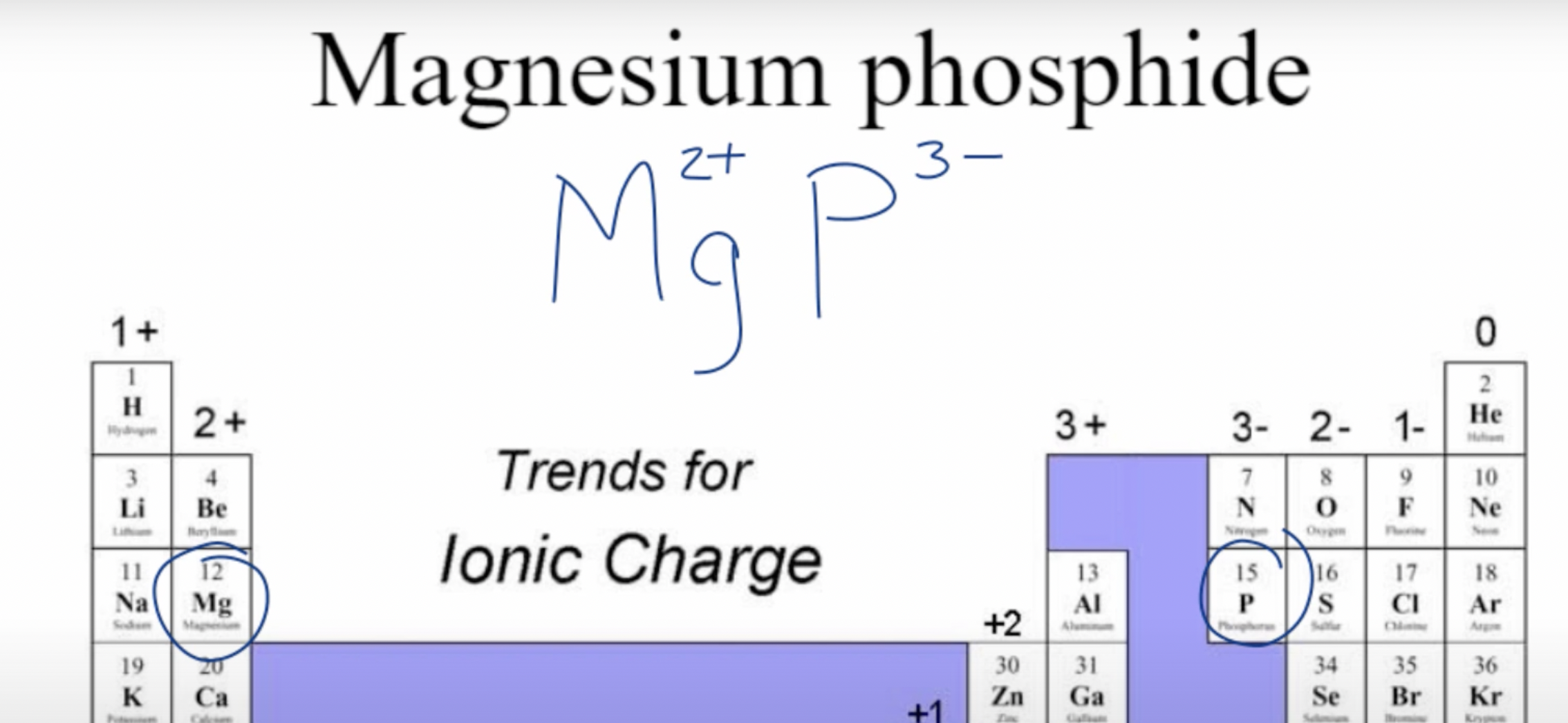
Lastly, we criss-cross the charges, and the final formula is:
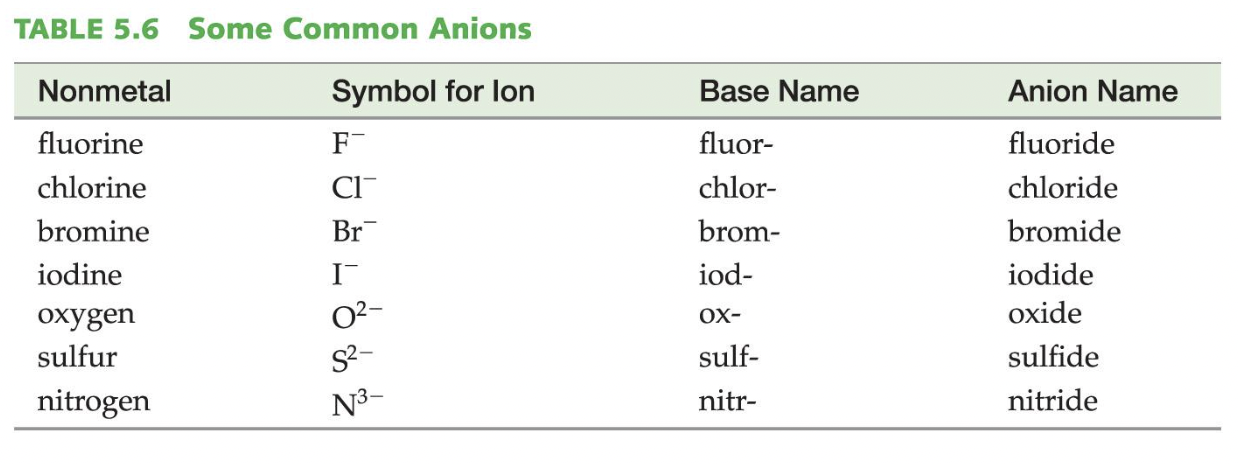
Writing the names of binary ionic compounds is even more simple because we just take the metal name, and then the base of the nonmetal name is added with the ending -ide.
For example, NaCl has two elements: Sodium and Chlorine → Sodium Chloride
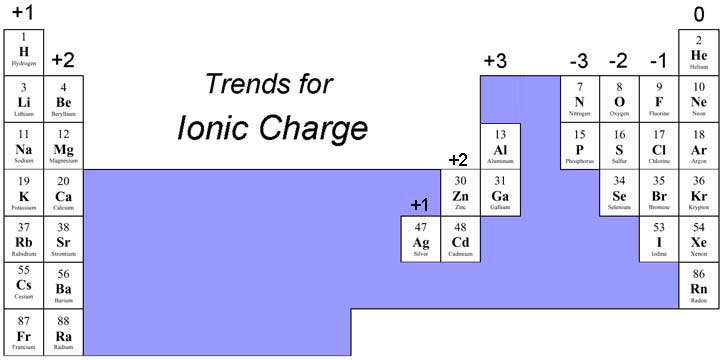
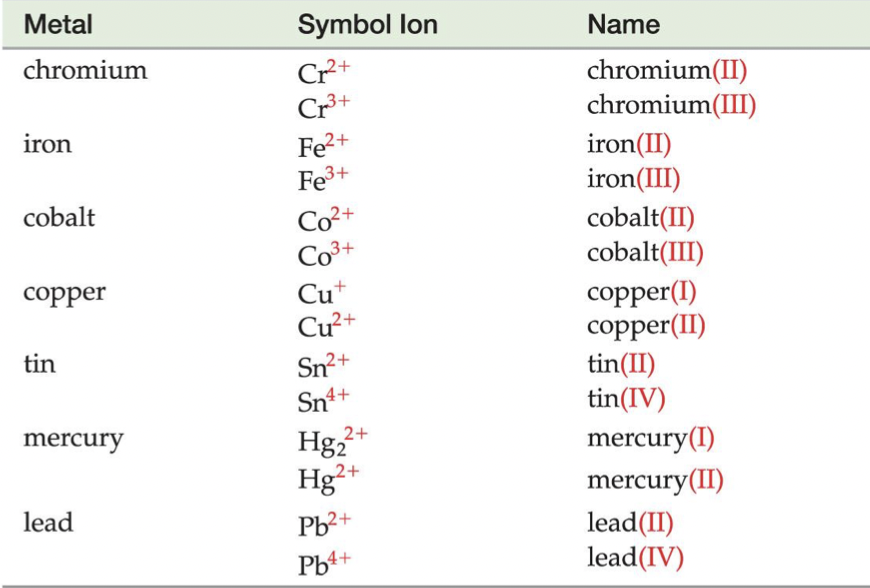
BUT… some metals have more than one charge, which is why they are not on the Ionic Trends chart, so when writing the name of a binary ionic compound with a metal with more than one ion, we write its Roman numeral in parenthesis to specify
7.2 Polyatomic Ionic Compounds
Polyatomic ionic compounds are different from binary ionic compounds because they have ions with more than one atom.
Binary Ionic: NaCl, both ions are composed of a single element
Polyatomic Ionic: NaOH, “OH-” is Hydroxide which is composed of the two elements Hydrogen and Oxygen
When naming a compound like this, we use the name of the polyatomic ion
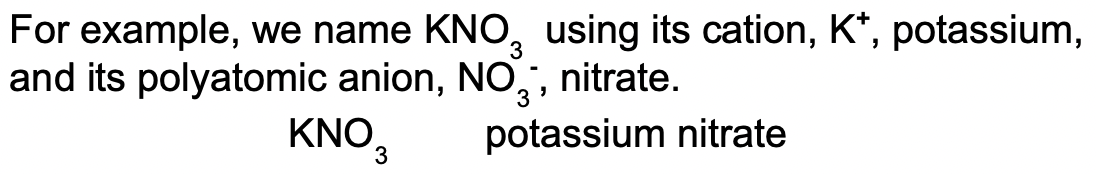
Oxyanions
These are the polyatomic ions with oxygen in their anions. According to how many oxygen atoms are in the ion, they will be named differently. When there are two ions in the series, the one with more oxygen atoms will have the ending -ate, and the fewer will have -ite.
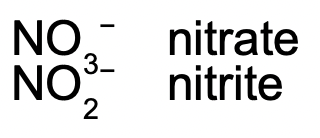
When there are more than two ions in the series, hypo- and per- are used.
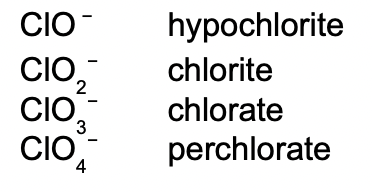
As can be seen above, hypo- will be given to the ion with the least number of oxygen atoms, while per- will be given to the one with the most.
A Mnemonic used for remembering the “-ates” (one more oxygen atom than -ite) is:

Note: Although this Mnemonic seems to provide just the -ates, you can figure out the others by adding or removing an oxygen atom
Common Ionic Charges to memorize
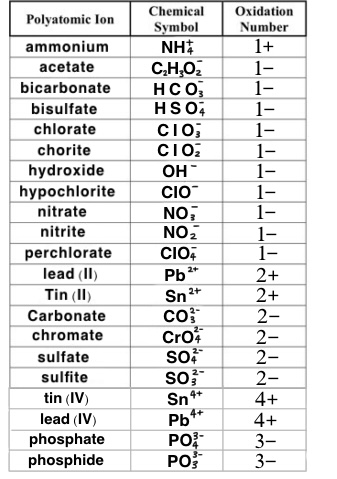
Example Problem: Copper (II) chlorite
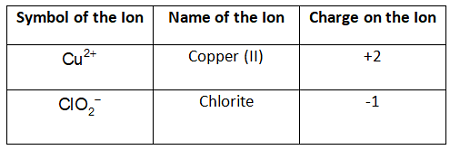
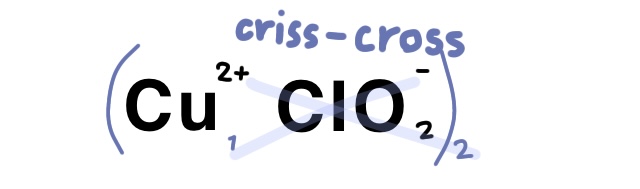
Answer:
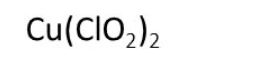
Example Problem: Strontium hydroxide
Strontium is in group 2A of the periodic table, meaning it will have a 2+ charge
Hydroxide is a polyatomic atom that has to be memorized, it is OH-.
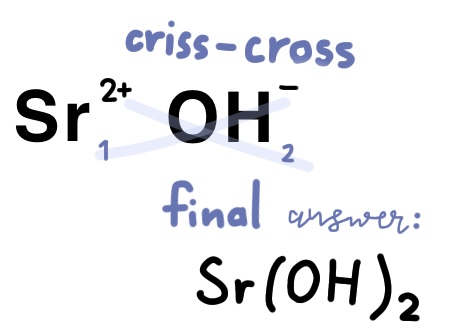
7.3 Covalents and Acids
A covalent or molecular compound is composed of two nonmetals, and we use prefixes to help name them. Just like when naming Binary Ionic compounds, we also add an -ide ending to the second element.

Naming Acids
Acids have hydrogen typically written first in their formula, followed by nonmetals. There are two main categories of acids: Binary acids (contain two elements) and Oxyacids (contain oxygen).
A Binary acid begins with the prefix hydro-, added onto the nonmetal base name, with the ending -ic. The word acid is then added at the end.
Example: HCl is hydrochloric acid, and HBr is hydrobromic acid
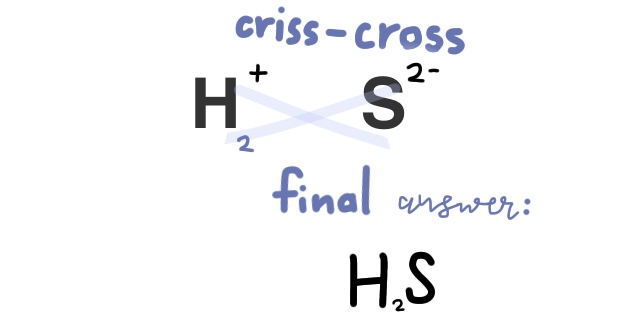
Practice problem: Write the formula for Hydrosulfuric acid
First, because it starts with hydro-, we know that this compound is just two elements, hydrogen, and sulfur. We find sulfur’s charge next, which is 2-, then balance it out.
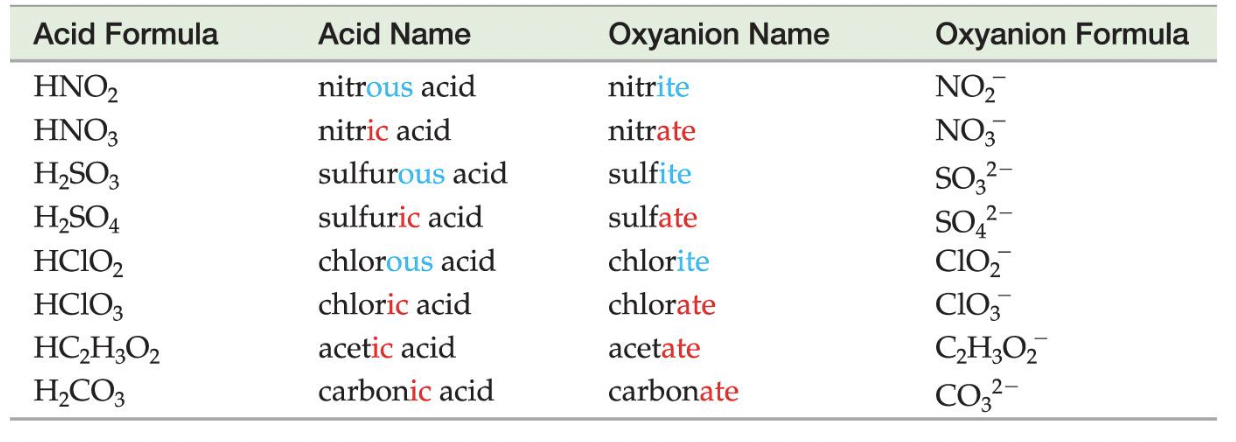
Oxyacids consist of hydrogen, and an oxyanion (refer to above).
Example: HNO3 contains the nitrate (NO3−) ion, H2SO3 contains the sulfite (SO32−) ion, and H2SO4 contains the sulfate (SO42−) ion
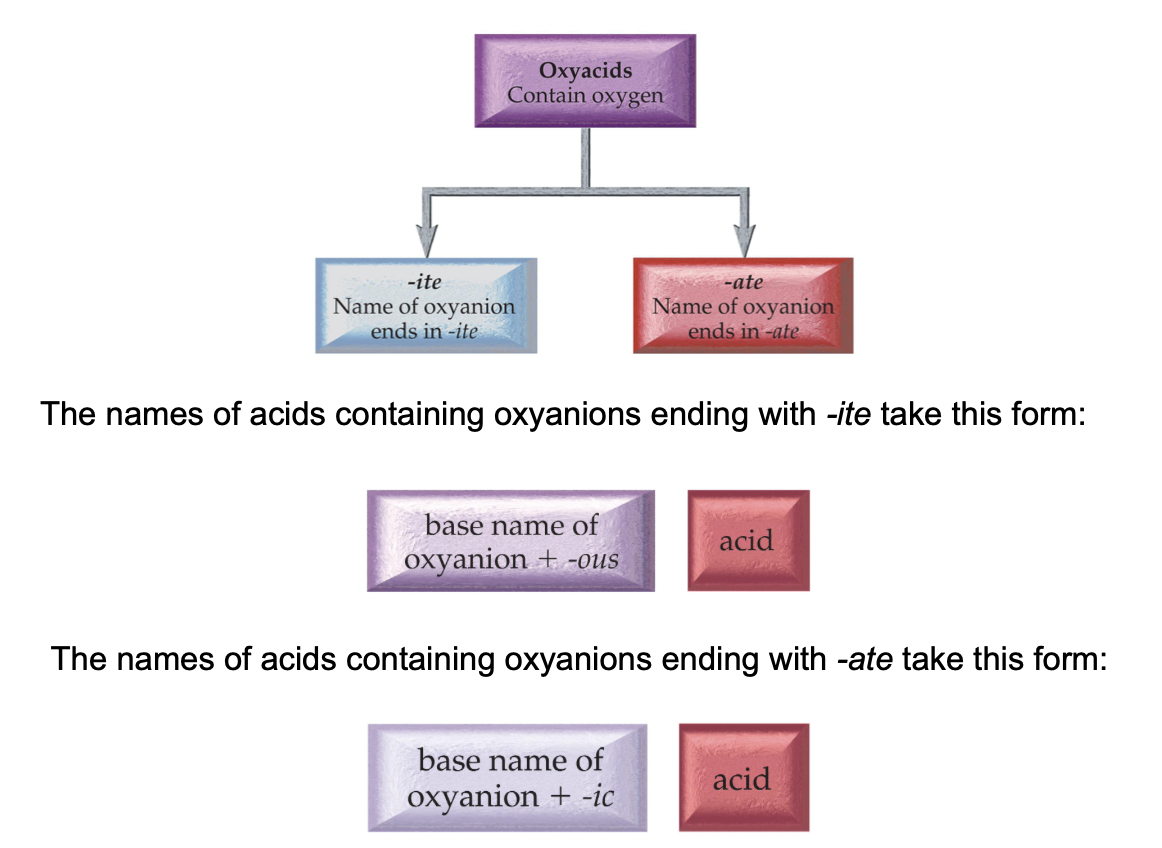
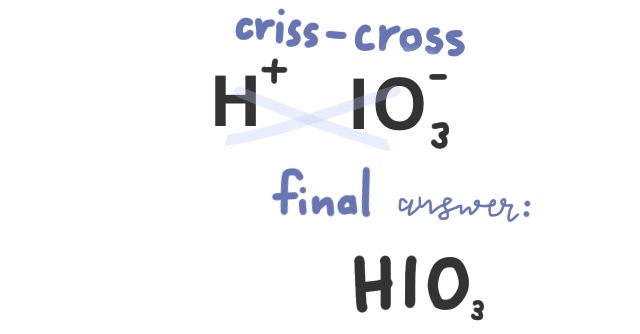
Practice Problem: Write the formula for Iodic acid
Since there is no prefix hydro-, we know this compound contains an oxyanion. Iodic has the ending stem -ic, meaning that iodate must be the polyatomic ion.