Unit 1: Atomic Structures & Properties
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
28 Terms
Pauli Exclusion
Atomic orbitals can hold at most 2 electrons
Coulombs
Larger charges and smaller distances result in a stronger force, while like charges repel and opposite charges attract
Aufbau principle
Electrons fill the lowest energy orbitals first
Hunds rule
When filling a subshell of orbitals with the same energy, electron will first occupy each orbital singly before any orbitals is doubly occupied. Thus minimizes electron repulsion and is more stable
Photoelectric spectroscopy
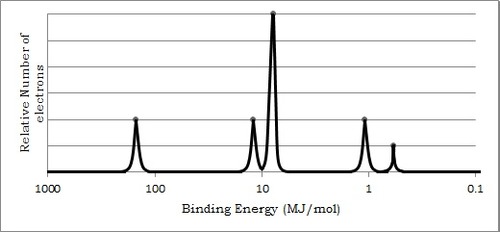
Atomic number
Number of protons
Atomic mass/ Mass number
Sum of protons and neutrons
Mass spectrum to determine Average atomic mass
Mass number × % expressed as a decimal
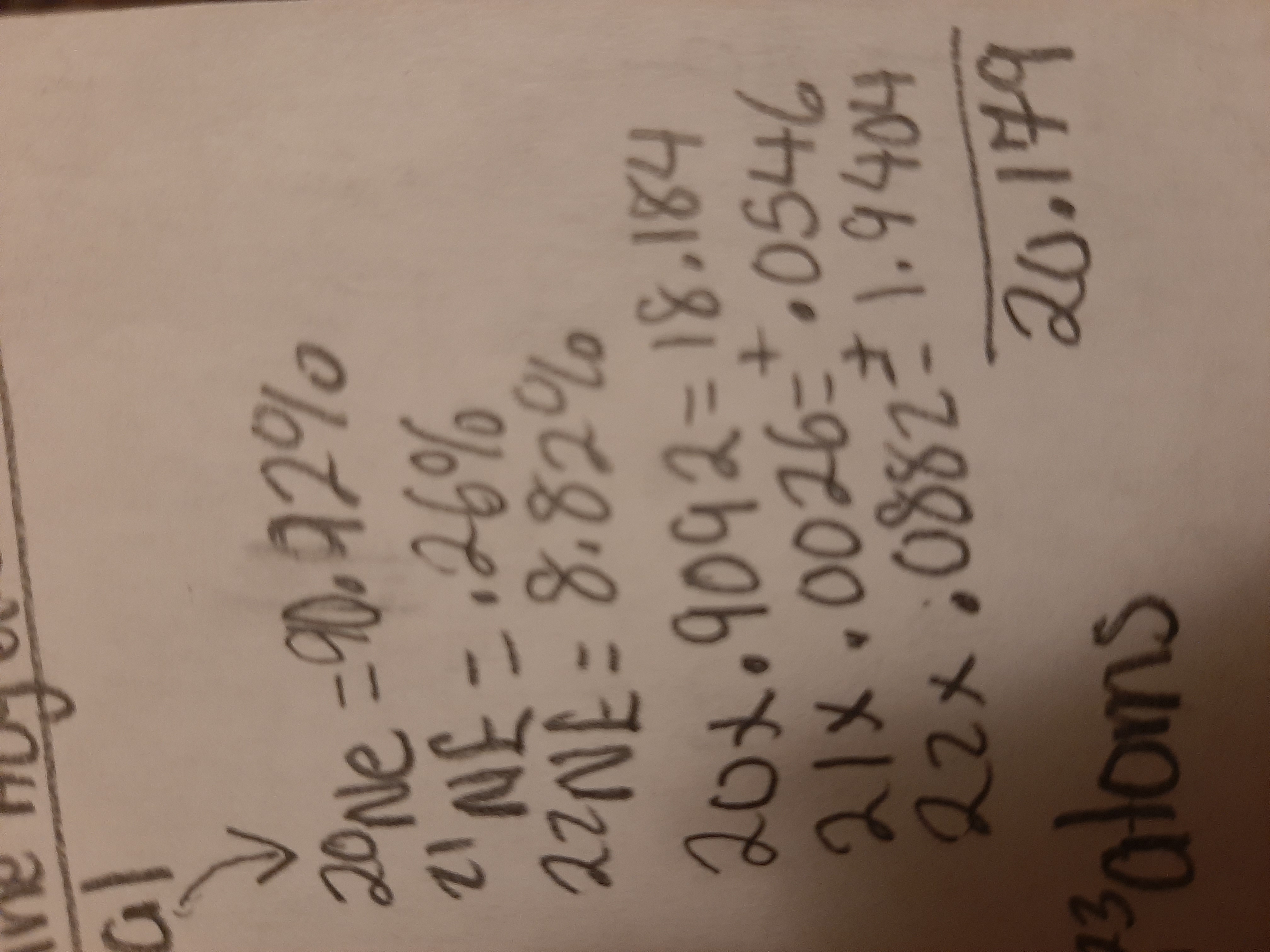
Pure substance
Only one type of matter (single element or compound)
Mixtures
Can be physically separated ( homogeneous & heterogeneous)
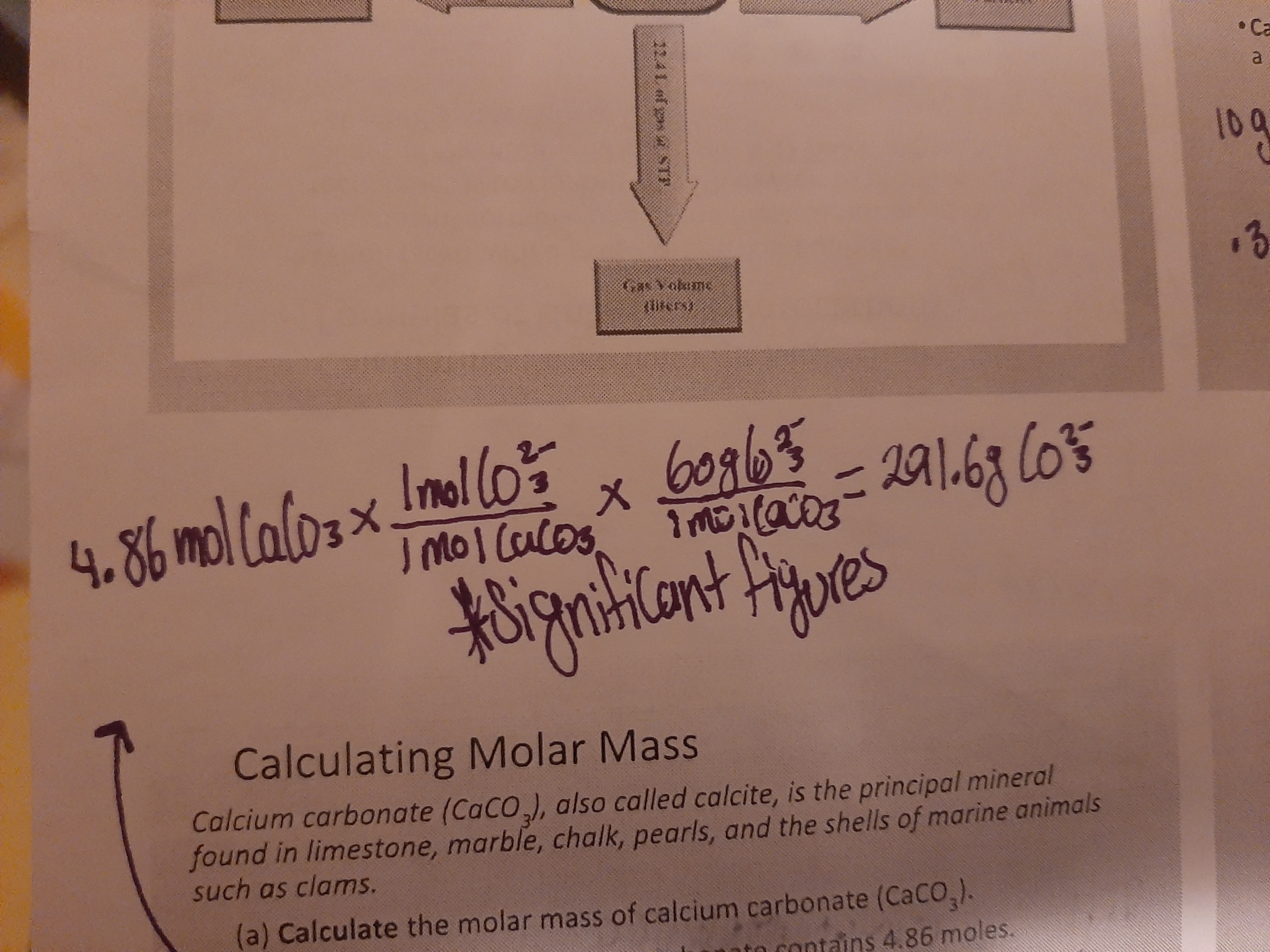
Determining moles of atoms
Divide given grams by molar mass to get moles then multiply moles by 6.02 × 10²³ atoms
Calculating mass of ions present
Given number of moles × 1 mol (ion)/1 mol (moles) × molar mass of CO3 = answer
Mass percent
Mass of desired element/ total mass of compound × 100%
Empirical/ molecular formula

Calculating empirical/molecular formula of unknown hydrocarbons

Atomic radius
Radius increase as you go down a group due to adding shells; radius decreases as you go right due to more protons pulling more protons closer to nucleus. Increase down and left
Ionic radius
Losing electrons means the protons pull on fewer electrons so the radius contracts; gaining electrons means more electron repulsion so radius expands
Isoelectronic species
Atoms/ions with same electron configuration
Ionization energy
Increases going up and right due to the energy required to remove an electron due to valence electrons being tighter and harder to pull
Electron Affinity
Energy associated with adding an electron opposite of ionization energy; increases up and right (no noble gases)
Electronegativity
How well an atom can attract electron density; more strongly attracting electron density means more electronegativity. Increases up and right (no noble gases) more nucleus can pull, more electronegative
Nonmetals
Tend to form anions, poor conductors of heat, tend to gain electrons
Metals
Tend to form cations, good conductors of heat and electricity, compounds formed between metals and non metals tend to be ionic
Group 1A: Alkali Metals (+1)
Reactions with water are exothermic, found only in compounds in nature, low densities and melting points
Group 2A: Alkaline Earth Metals (+2)
Less reactive with water than Alkali, reactivity tends to increase as you go down the group, have higher densities and melting points than Alkali
Group 6A: (Chalcogens)
Contains multiple Allotropes (different forms of the same element in the same state)
Group 7A: Halogens (-1)
Typical non metals, react directly with metals
Group 8A: Noble Gases
Large ionization energy, relatively unreactive