Drug design: Drug Targets
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
What are some examples of mammalian molecular targets?
- Receptors and Ion channels, proteins.
- Enzymes to catalyse intracellular processe.
- Nucleic acids to disrupt or induce replication and transcription.
What are some examples of non-mammalian molecular targets?
- Chemical targets to neutralise or disrupt membranes.
- Bacteria, fungi and viruses.
How are antagonists similar and dissimilar to agonists?
They feature the same functional group as the agonist so they can interact with the receptor however are larger to allow additional interactions with the receptor.
What are receptors?
Proteins embedded in plasma membrane and act as a relay to pass info to intracellular second messengers.
What are agonists?
First messengers that activate a second messenger through a conformational change in the receptor and are unchanged after binding to the receptor.
What is the primary structure of a protein responsible for?
Produce a protein backbone and are responsible for determining the type and strength of bonding interactions with drug molecules.
Describe the secondary structure of proteins.
Formed by hydrogen bonds between peptide backbones leaving residues. Forms α‐Helix and β‐sheet.
Describe the tertiary structure of proteins.
The most important structure for drug action and is achieved by additional interactions between side chain residues. Folding brings diverse amino acids into close proximity allowing binding site.
What are the types of bonds involved in drug-receptor interactions?
- Covalent
- Ionic
- Hydrogen bonds
- Van der waal forces.
Describe covalent bonds in drug receptor interactions.
- Rarely seen in pharmacodynamic receptors and irreversibly bind to receptors to block them.
- They are important in drug - DNA interactions and in enzymes.
- Very strong.
Describe ionic bonds in drug receptor interactions.
- Allow drug to bind and also dissociate to receptor.
- Caused by mutual attraction between the receptor and drug based on opposing charges.
- Medium strength.
Describe hydrogen bonds in drug receptor interactions.
- Essential in drug-receptor interactions.
- Caused by dipole interactions.
- Require many as too weak alone.
Describe Van Der Waal forces in drug receptor interactions
- Occurs with neutral carbons
- Caused by fluctuation in electron distribution.
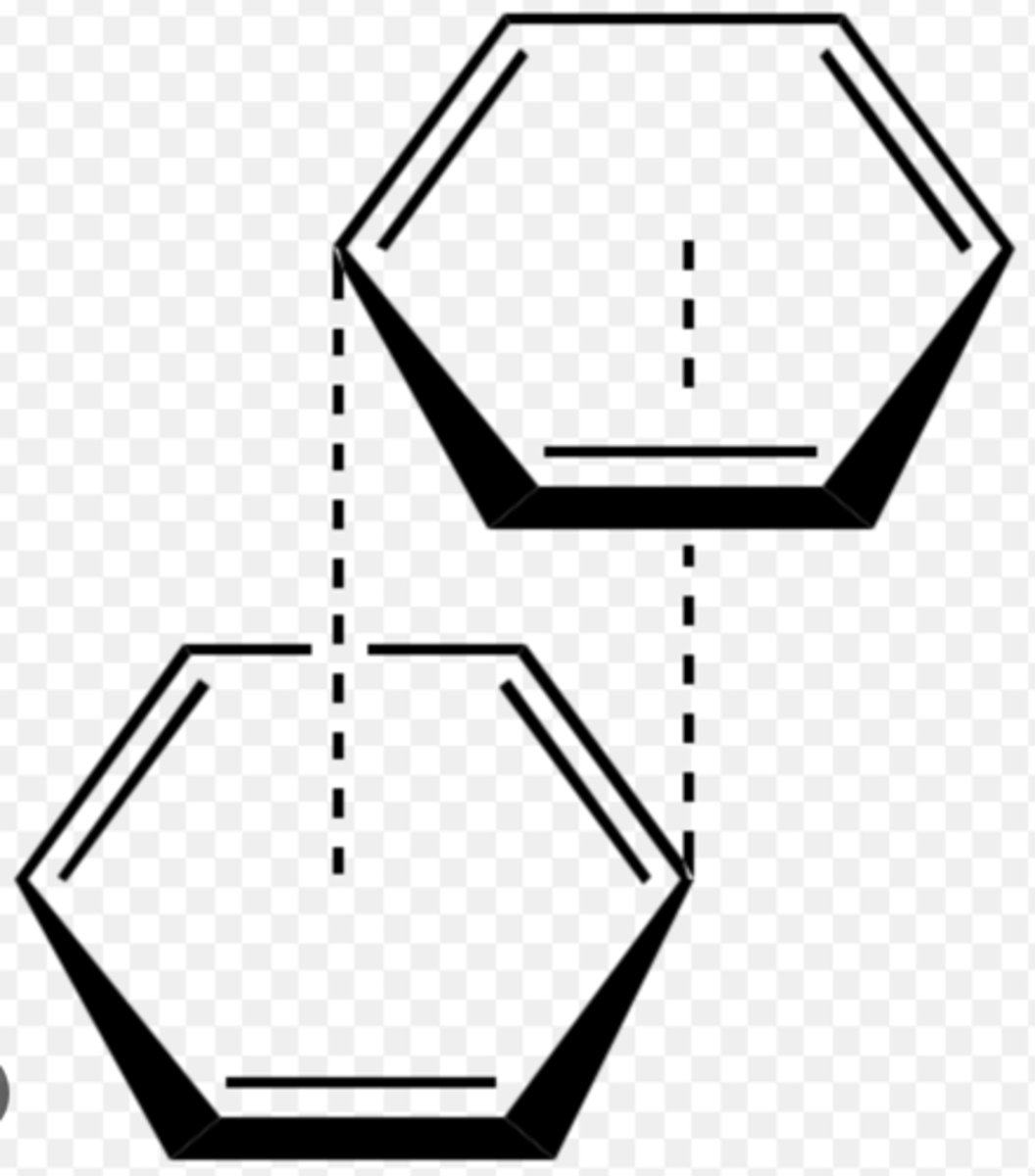
- Causes pi-pi stacking in aromatics.
- Alkyl residues fit into hydrophobic pockets formed by aromatic residues.
What is pi-pi stacking?
Aromatic π systems bind face to face with one another and involve a combination of dispersion and dipole-induced dipole interactions.