AP Chem Unit 7 Chemical Kinetics
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
Collision Theory
that bimolecular reactions occur when two correctly oriented molecules collide with sufficient energy.
For the bonds to form, the electrons in the atoms must be rearranged.
Further, if A and BC have filled shells of electrons they will repel one another.
3 Conditions:
The reactants must collide.
The reactants must align properly to break and form bonds.
The collision must provide the energy needed to “activate” the reaction.
Steric Factor
fraction of collisions with the proper orientation
Activation Energy
a specific amount of energy to start breaking the bonds in the reactants.
Reaction Rate
change in the concentration of a reactant or a product with time (M/s).
Can use ratios in moles in a chemical equation
Rates are Related to each other, Not Equal

Factors Affecting Reaction Rate
Number of reactants
More reactants/bonds → longer times
Surface Area
Increased Surface area → increased reaction rate → decreased time
Concentration of Reactants
More reactants →more particles → more collisions → increased Rxn rate
Temperature
Increased Temp → increased energy → increased collisions → increased Rxn rate
Catalyst
compounds affecting the rate of reaction.
Decrease activation energy
Differential Rate Law
Based solely on Experimental Data
x and y are coefficients
k is the rate constant
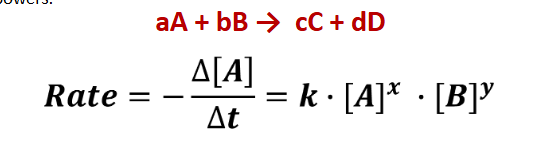
Reaction Order
sum of powers to which all reactant concentrations appearing in rate law are raised
x + y
won’t usually see greater than 3

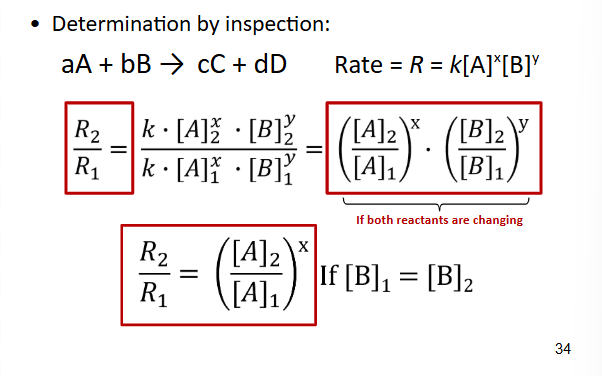
Meaning of Zero Order
If the concentration of your reactant changes, but the rate remains constant
Meaning of First Order
If the concentration of your reactant doubles and the rate also doubles
Meaning of Second Order
If the concentration of your reactant doubles and the rate quadruples
Rate law & Reaction Order
Types of Reaction Rates
Instantaneous Rate — One moment in time
Initial Rate — Right at start of reaction
Average Rate — Over interval (Slope of secant line between two points on the curve)
Integrated Rate Laws
The mathematical equation that relates the concentration of a reactant or product to the time it takes for a reaction to occur.
Cam calculates the concentration(reactant and product) or time for reaction
Need data which is linear (k) for the equation
Zero Order Integrated Rate Law
Mainly seen for enzymes
Rate is Independent of the reactant concentration

Zero Order Reactions Half Life
Value is constant
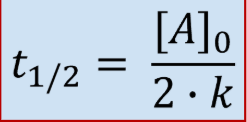
First Order Integrated Rate Law
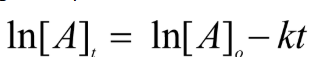
First Order Half Life
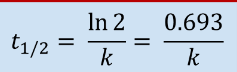
Second Order Integrated Rate Law
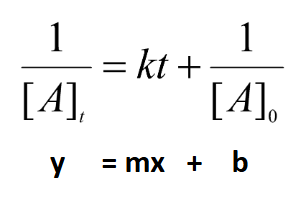
Units for Integrated Rate Laws
Based on orders.
Is 1/m(order-1)s
Ex.
0 order: s
1st order: 1/s
2nd order: 1/ms
Second Order Half Life
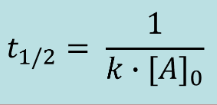
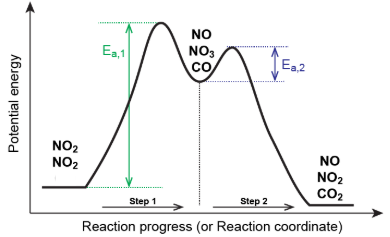
Multistep Reaction Energy Profile
a visual representation of the energy changes occurring during a chemical reaction that happens in multiple steps
Peaks = Steps
Valleys = Intermediates
Highest Activation Energy is the rate-limiting step
Catalysis
When A catalyst decreases activation energy —> increasing rate of a reaction
more collisions will have the needed energy to form products!
Types of Catalysis
Important Ones:
Homogeneous Catalysis — Catalyst is completely mixed with reactants, maximizing collisions between reactants and catalyst
Heterogeneous Catalysis — Catalyst exists in a different phase than the reactants, often as a solid surface where the reaction occurs.
Enzyme Catalysis — enzymes, which are protein molecules, act as catalysts with high specificity for certain reactions.
Others:
Autocatalysis — when the reaction where one of the products acts as a catalyst for the reaction itself, accelerating its rate.
Acid-Base Catalysis — where an acid or a base increases the rate of the reaction by facilitating the transfer of a proton (H+) between species
Electrocatalysis — type of catalysis that occurs at an electrode surface, where the catalyst facilitates electron transfer during an electrochemical reaction.
Heterogeneous Catalysis
Usual Steps:
Adsorption of the reactant(s) onto the surface of the catalyst.
Activation of the adsorbed reactant(s).
Reaction of the adsorbed reactant(s).
Desorption of product(s) from the surface of the catalyst.
often termed “Surface Catalysis” because the reactants interact with an inert surface during the reaction
Enzymes
Characteristics:
Specificity: Enzymes are highly selective catalysts
Recyclability: Enzymes are not destroyed during a reaction, can be reused
Insolubility: Enzymes are insoluble.
Saturation point: Enzymes have a saturation point, where activity ceases once all the enzymes are occupied by substrate molecules.