Magma diversification
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
Magma Differentiation:
-Any process by which a magma is able to diversify and produce a magma or rock of different composition.
Two essential processes:
1. Create a compositional difference in one or more phases
2. Preserves the chemical difference by segregating (or fractionating) the chemically distinct portions:
Most common: differentiation involving the physical separation of phases in multi-phase systems
-The effectiveness depends upon contrasts in physical properties such as density, viscosity, diffusivity, and size/shape
-The energy is usually thermal or gravitational
-The phases that are fractionated in magmatic systems can be either liquid-solid, liquid-liquid, or liquid-vapor
Partial Melting
Separation of a partially melted liquid from the solid residue
-Separation of a partially melted liquid from the solid residue requires a critical melt %
-For mafic melts, this may be as low as 1%.
-For more viscous felsic melts it is higher (they have a higher dihedral angle).
-Sufficient melt must be produced for it to
Form a continuous, interconnected film
Have enough interior volume that it is not all of it is adsorbed to the crystal surfaces
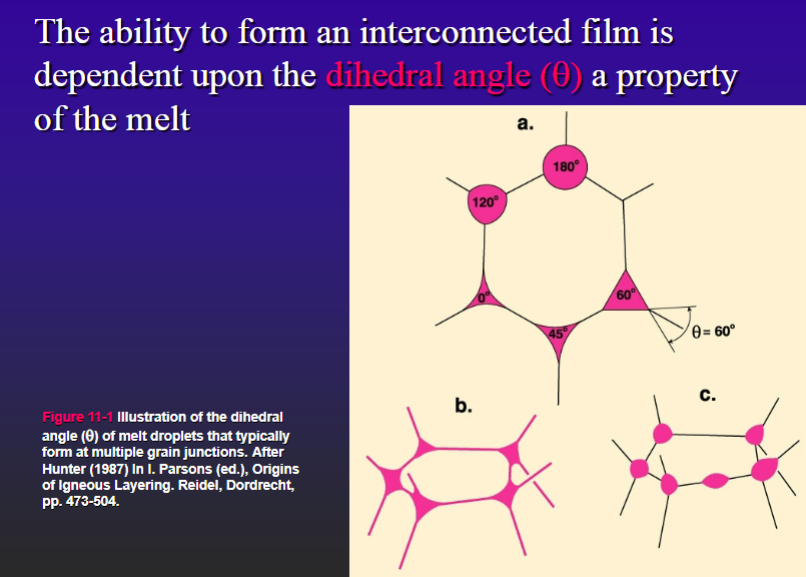
critical melt fraction
-The critical melt fraction (or rheological critical melt percentage, RCMP): % melt at which a crystal-dominated, high viscosity granular framework gives way to a melt-dominated, lower viscosity suspension (crystal mush)
-RCMP varies with T, viscosity, X
The separation of melts from residuum is facilitated by:
-Gravitational effects (buoyant liquid)
-Filter pressing, or compaction, of crystal mush
-Shear - the RCMP drops considerably (changing T, viscosity, X)
Estimated range of RCMF
Basalt:1-7% for basalt/peridotite
Silicic magmas:15-30%
Partial melting may be responsible for the generation of a variety of magmas (Tholeiitic and alkaline) via partial melting of mantle lherzolite
What is the most dominant mechanism by which most magmas, once formed, differentiate?
Fractional Crystallization:
-Separation of crystals from liquid
-Gravitative settling or flotation play a significant role
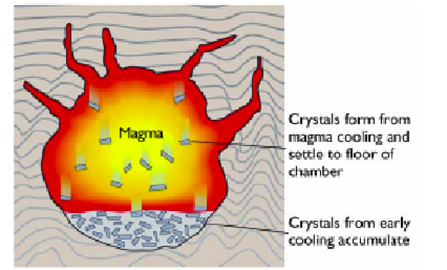
Gravity settling or floatation:
-The differential motion of crystals and liquid under the influence of gravity due to their differences in density- gravitational differentiation
-Forms cumulate plutonic rocks
-Crystal sinking experiments
-E.g dunite, pyroxenite, wehrlite (ol+cpx) or cumulate gabbros and anorthosites
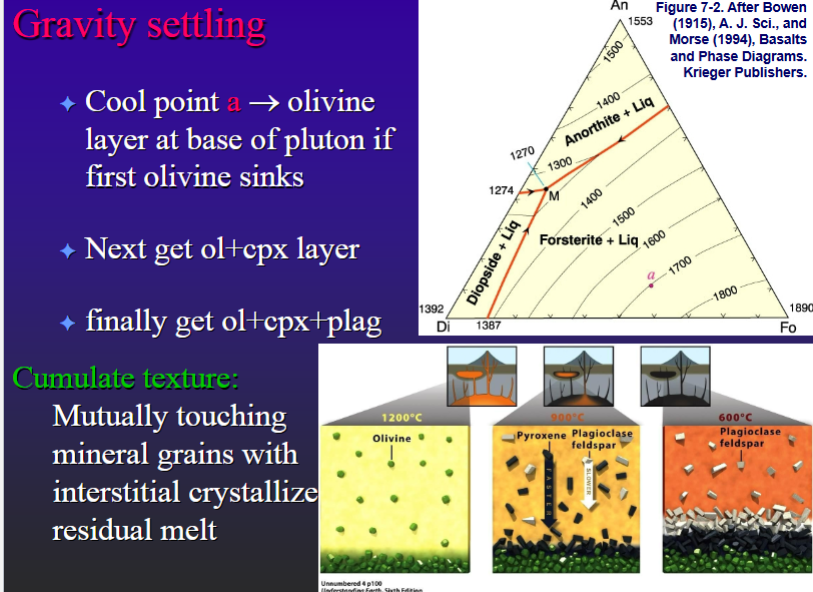
Gravity settling
Cool point a → olivine layer at base of pluton if first olivine sinks
Next get Ol+CPX layer
Finally get ol+cpx+plag
Cumulate texture: Mutually touching mineral grains with interstitial crystallize residual melt
Two other mechanisms that facilitate the separation of crystals and liquid
1. Compaction
Crystal settling squeezes melt out of crystal mush zone
1. Flow segregation (localized)
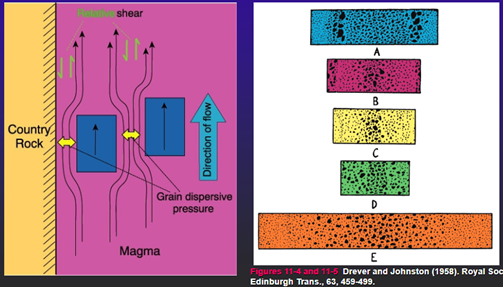
Compositional Convection and In Situ Differentiation Processes
-In-situ: crystals don’t sink/move
-Typically involves:
Diffusion
Convective separation of liquid and crystals
-Helps explain some chemical trends, particularly apparent “over enrichments” of highly incompatible elements in the boundary layer
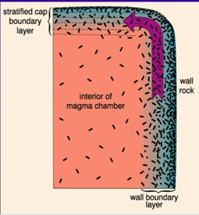
Langmuir Model:
-Thermal gradient at wall and cap → variation in % crystallized
-Compositional convection → evolved magmas from the boundary layer to cap (or mix into interior)
Be familiar with stokes law (know the formula, what its variables are and strengths/weaknesses in describing crystal movement in a magma .
Stoke’s Law: Model settling velocities for spherical particles in Newtonian fluid (no yield stress)
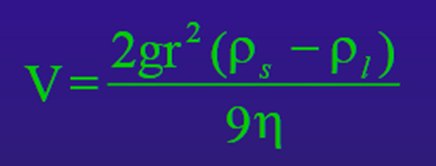
V= the settling velocity (cm/sec)
G= the acceleration due to gravity (980 cm/sec^2)
r= the radius of a spherical particle (cm)
Ps= the desnity of the solid spherical particle (g/cm^3)
P1= the density of the liquid (g/cm^3)
N= the viscosity of the liquid (0.1 Pa.s= 1 poise)
Stoke’s Lae is overly simplified!!!!!
-Crystals are not spherical
Tabular, acicular, and platy minerals will settle with slower velocities, but it is difficult to determine exactly how much slower
-Only basaltic magmas very near their liquidus temperatures behave as Newtonian fluids
Once begin to crystallize, crystals develop a significant yield strength that must be overcome before any motion is possible
e.g. In order to overcome this resistance an olivine crystal must have been several centimeters in diameter!
Yield strength is considerably higher for cooler and more silicic liquids
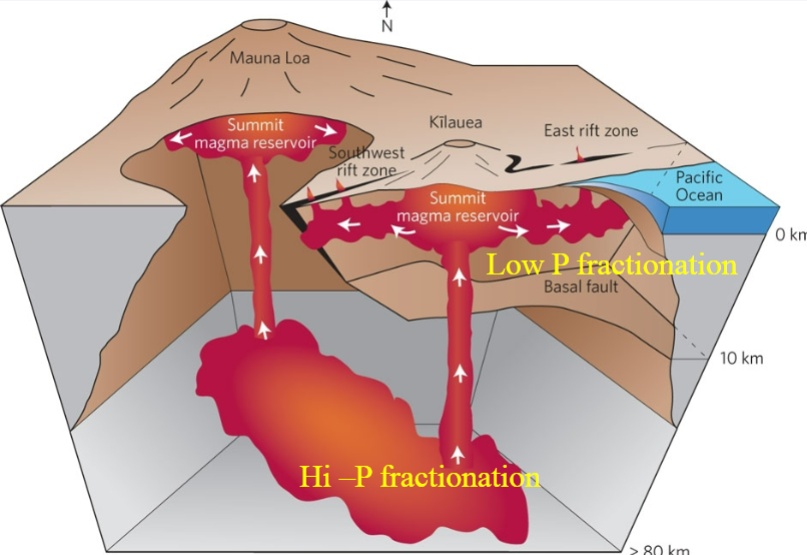
Polybaric Fractional Crystallization
-Phases crystallizing change with changes in pressure:
Order of crystallization changes
Stability of phases changes (hi-P garnet…)
-Primary or primitive melts may first crystallize at great depths and high P (ex. Base of crust) and then the evolved melts will fractionally crystallized at shallow depths (low pressure) in the upper crust
Shift of the eutectic point with pressure will cause the quantity of the liquidus phases to vary
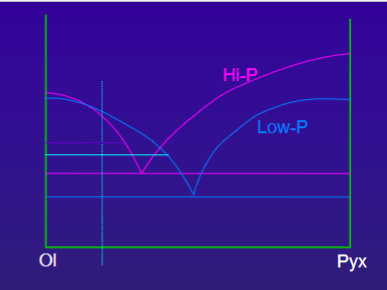
How does Ol/liquid change from high-P to low-P crystallization
Expansion of olivine field at low pressure causes an increase in the quantity of crystallized olivine
Thus, the amount of olivine that crystallizes with a rising basaltic magma will be greater that the amount that forms during isobaric crystallization
Volatile Transport
Vapor released by heating of hydrated or carbonated wall rocks
Late-stage fractional crystallization
-fractional crystallization enriches late melt in incompatible volatiles and non-lithophile elements (chalcophile)
-many concentrate further in the vapor phase
-particularly enriched with resurgent boiling (melt already evolved when vapor phase released)
-get a silicate-saturated vapor + a vapor-saturated late derivative silicate liquid - forms pegmatites
The vapor phase concentrates volatile constituents such as H2O, CO2, S, CL, F, B, and P, as well as a wide range of incompatible and chalcophile elements (esp LILS)
Volatile release
-Volatile release raises liquidus temperature → forms fine groundmass and porphyritic texture
-May increase directed P - fracture the roof rocks (explosive phase of magmatism)
-Vapor and melt escape along fractures as dikes
Silicate melt → quartz and feldspar → small dikes of aplite
Vapor phase → dikes or miarolitic pods of pegmatite (due to poor nucleation and high diffusivity in the water-rich phase)
Mineralization (e.g. gold, silver, lead, copper)
Porphyry Copper Deposits
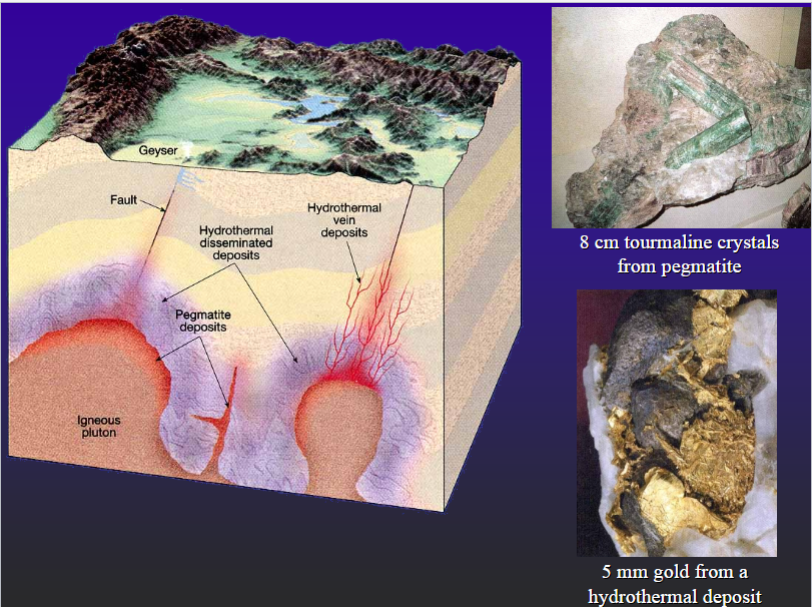
Pegmatitic Dike
Coarse crystals in core – finer on contact

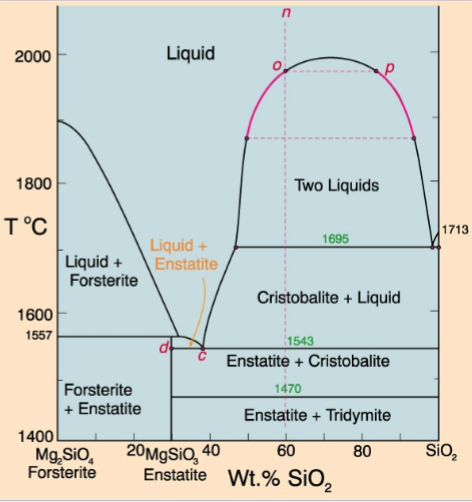
4. Liquid Immiscibility
Liquid immiscibility in the Fo-SiO22 systemsystem
Could LI generate granitic (Si-rich) liquids from more basic liquids?
Problematic:
1. Too hot
2. Alkalis
The effect of adding alkalis, alumina, etc. is to eliminate the solvus completely
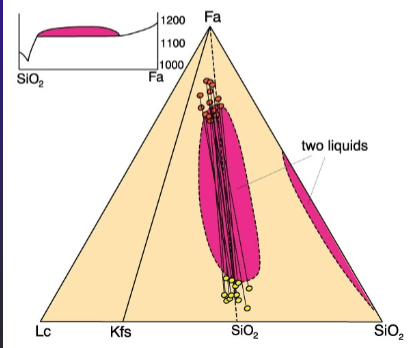
Some Examples of Immiscible Liquids in Natural Systems
Late silica-ric immiscible droplets in Fe-rich tholeiitic basalts
Sulfide-silicate immiscibility (massive sulfide deposits)
Carbonatite-nephelinite systems
What is AFC and why is it more than just two unrelated processes operating simultaneously?
Assimilation
-Incorporation of wall rocks (diffusion, xenoliths or enclaves, melt)- stoping, zone melting
-Assimilation by melting is limited by the heat available in the magma
Hmelt+Hxtal (latent heat of crystallization)
2.5 g of magma needs to crystallize à 1 g of assimilating melt!
Combined process – fractional crystallization + assimilation (AFC! After dePaolo
Initial partial melting during assimilation adds small % of siliceous and incompatible trace element enriched melts to the parent magma
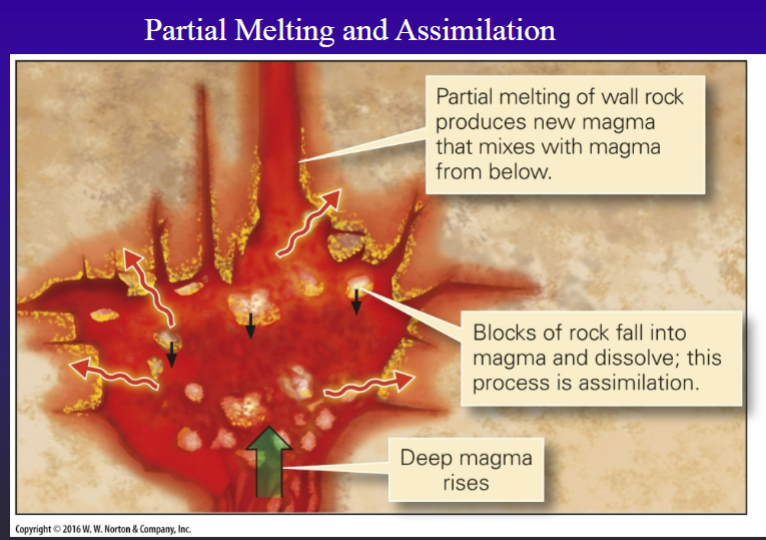
Detecting and assessing assimilation:
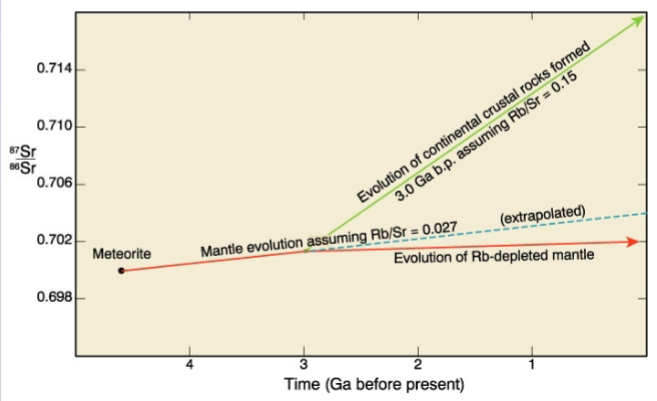
Isotopes are generally the best
-Continental crust becomes progressively enriched in 87Sr/86Sr and depleted in 143Nd/144Nd
U-Th-Pb system as an indicator of continental contamination is particularly useful 207Pb/204 Pb and 206Pb/204Pb ratios are considerably higher in the older continental crust than in the mantle or in mantle-derived melts
Because the amount of Pb in the basalts is relatively small, it is very susceptible to contamination by crustal Pb
Mixed Processes
-May be more than coincidence: two processes may operate in conjunction (cooperation?)
-AFC: FX supplied the necessary heat for assimilation
-fractional crystallization + recharge of more primitive magma
-will change isotopic ratios depending on concentration of isotopic element and mass of each melt
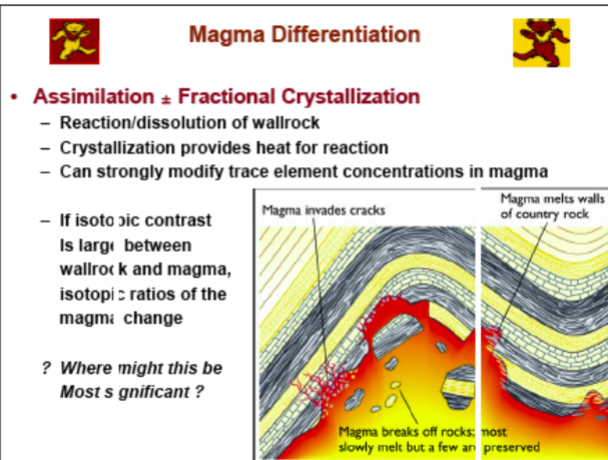
Assimilation + Fractional Crystallization
-Reaction/dissolution of wall rock
-Crystallization provides heat for reaction
-Can strongly modify trace element concentrations in magma
-If isotopic contrast is large between wallrock and magma, isotopic ratios of the magma change

5. Magma Mixing
-End member mixing for a suite of rocks
-Variation on Harker-type diagrams should lie on a straight line between the two most extreme compositions
Mixing + crystallization= curved lines
Mixing of 2+ types of melt= Curved lines
Mixing can be detected:
-structurally
-geochemically
-mineralogically
-isotopically
-texturally
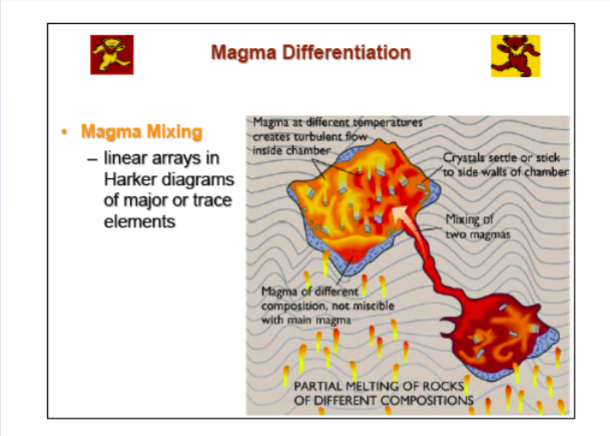
Magma Mixing
Magma mixing is most evident in cases the magmas are quite different, such as basalts
and intermediate or silicic types
Due to the large differences in the physical properties of the contrasting magmas, the
degree of mixing of these comingled magmas is usually not extensive

Magma differentiation and diversification
-Partial melting
-Fractional crystallization (isobaric/polybaric)
Gravity/compaction/flow segregation
-Volatile transport
-Liquid Immiscibility
-Magma mixing and assimilation
Tectonic-Igneous Associations
-Mid-Ocean Ridge Volcanism
-Ocean Intra-plate (Island) volcanism
-Continental Plateau Basalts
-Subduction-related volcanism and plutonism
Island Arcs
Continental Arcs
-Granites (not a true T-I Association)
-Mostly alkaline igneous processes of stable craton interiors
-Anorthosite Massifs