Chapter 12: Infrared Spectroscopy and Mass Spectrometry
1/69
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
70 Terms
What is one of the most important tasks of organic chemistry?
determination of organic structures
What can chemical test suggest?
it can suggest the functional groups and narrow the range of possible structures before the physical properties are used to make an identification
Chemical test are....
inefficient
unlike chemical test, most spectroscopic techniques are what?
non-destructive; that is, the sample is not destroyed
What are four different types of spectroscopic and related techniques?
1. Infrared (IR) spectroscopy
2. Mass spectrometry (MS)
3. Nuclear magnetic resonance (NMR) spectroscopy
4. Ultraviolet (UV) spectroscopy
Spectroscopic and related techniques:
1. Infrared (IR) spectroscopy-
-covered in this chapter, observes the vibrations of bonds
-provides evidence of the functional groups present
Spectroscopic and related techniques:
2. Mass spectrometry-
-is not a spectroscopic technique, because it does not measure absorption or emission of light.
-bombards molecules with electrons and breaks the molecules into fragments
-analysis of the masses of the fragments gives the molecular weight, possibly the molecular formula, and clues to the structure and functional groups. Less than a milligram of sample is destroyed in this analysis
Spectroscopic and related techniques:
3. Nuclear magnetic resonance (NMR) spectroscopy-
-observes the chemical environments of the hydrogen atoms or the carbon atoms
-provides evidence for the structure of the alkyl groups and clues to the functional groups
Spectroscopic and related techniques:
4. Ultraviolet (UV) spectroscopy-
-observes electronic transitions
-provides information on the electronic bonding in the sample
What are five examples of electromagnetic radiation?
1. Visible light
2. Infrared light
3. Ultraviolet light
4. Microwaves
5. Radio waves
What do all the types of electromagnetic radiations have in common and how are they different?
Common: they all travel at the speed of light (3x10^10 cm/sec)
Different: they differ in frequency and wavelength
What is frequency?
(V, "nu") of a wave is the number of complete wave cycles that pass a fixed point in a second. It is usually given in Hertz, meaning "cycles per second"
What is the wavelength?
(lambda) is the distance between any two peaks (or any two troughs) of the wave
What is the relationship between wavelength and frequency?
-they are inversely proportional

electromagnetic waves travel as what?
photons, which are massless packets of energy
what is the equation of the energy of a photon?
-the energy of a photon is proportional to its frequency and inversely proportional to its wavelength
-h is planck's constant, 6.62x10^-37 kjsec
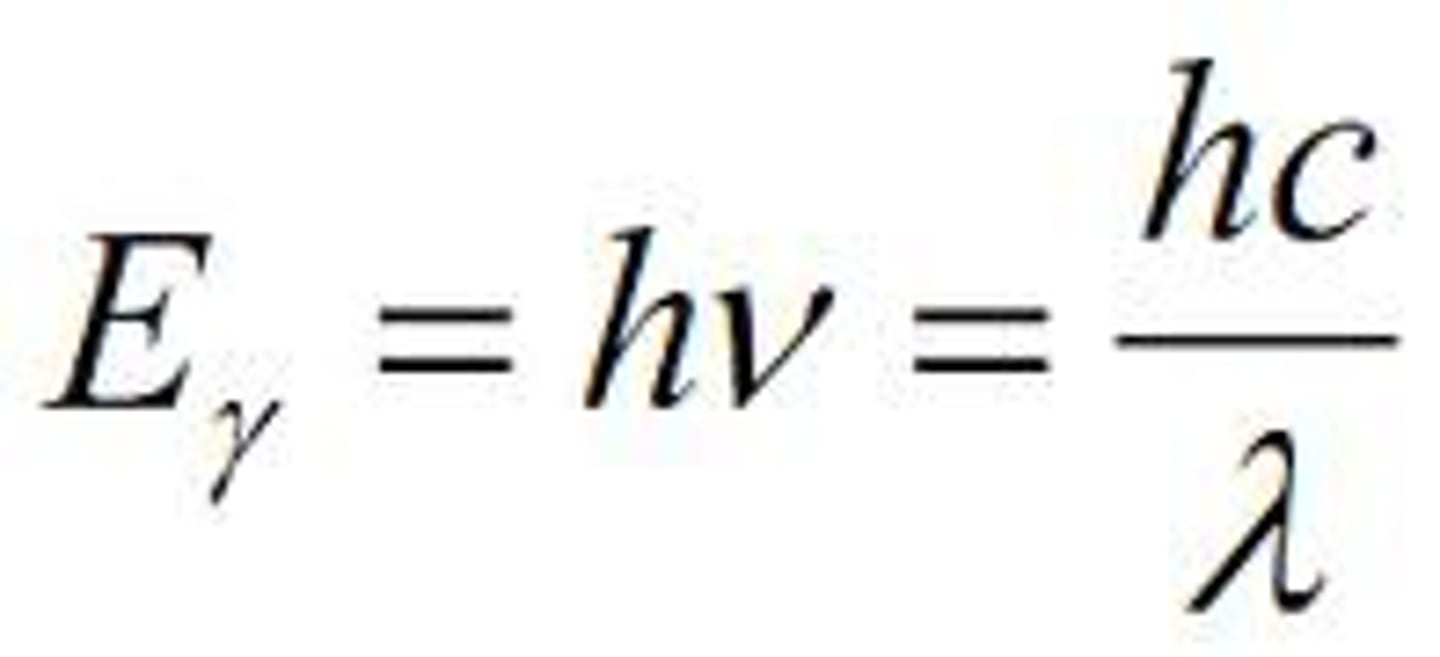
What is the electromagnetic spectrum?
the range of all possible frequencies, from zero to infinity
Electromagnetic Spectrum:
Higher frequency=
shorter wavelength
Electromagnetic Spectrum:
Lower frequency=
longer wavelength
What is the order of electromagnetic radiations from highest frequency to lowest frequency?
1. Gamma rays
2. X rays
3. Vacuum UV
4. Near UV
5. Visible light
6. Infrared (IR)
7. Microwave
8. Radio
Molecular Effects:
Gamma rays, X rays, Vacuum UV-
ionization
Molecular Effects:
Near UV, visible light-
electronic transitions
Molecular Effects:
Infrared (IR)-
molecular vibrations
Molecular Effects:
Microwave-
rotational motion
Molecular Effects:
Radio-
nuclear spin transitions
what is the equation for the energy of photon?
E=hv (frequency in hertz) = hc/lambda (wavelength in centimeters)
What is the equation regarding wavelength and frequency?
vlambda= c (v= frequency in hertz; lambda= wavelength in centimeters; c= speed of light)
The infrared region of the spectrum corresponds to frequencies from what?
just below the visible frequencies to just above the highest microwave and radar frequencies: wavelengths of about 8X10-5 cm to 1x10-2 cm
Infrared photons do not have enough energy to do what? But what can they do?
-they do not have enough energy to cause electronic transitions
-they can cause groups of atoms to vibrate with respect to the bonds that connect them
Infrared Photons:
Vibrational transitions correspond to...
distinct energies in which molecules absorb infrared radiation only at certain wavelengths and frequencies
The position of an infrared band can be specified by what?
its wavelength, which is measured in microns
What is the most common method for specifying IR absorptions?
-wavenumbers (cm-1); the reciprocal of the wavelength
-the wavenumber is proportional to the frequency (v) of the wave, so it is also proportional to the energy of a photon of this frequency (E=hv)
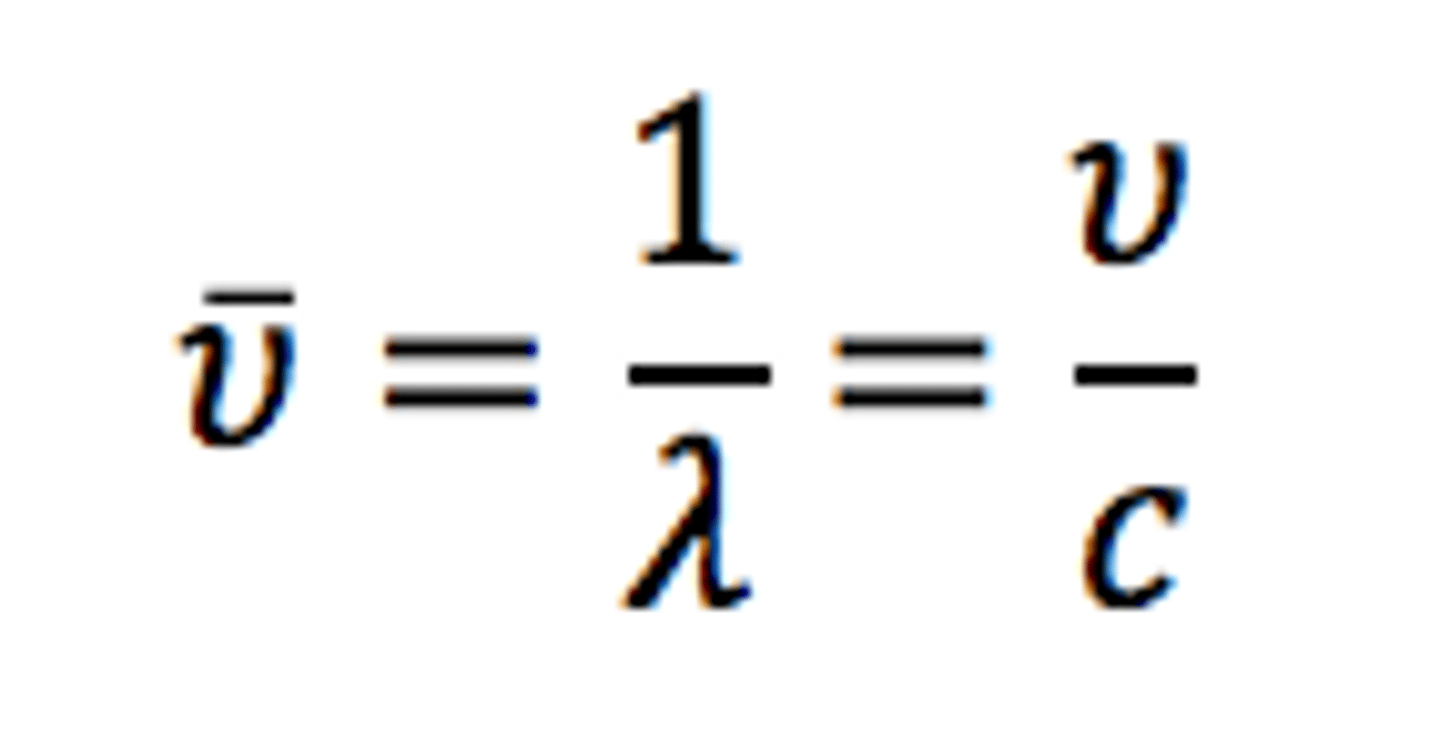
Molecular Vibrations:
The frequency of a stretching vibration depends on what?
-it depends on the masses of the atoms and the stiffness of the bond.
-heavier atoms vibrate more slowly than lighter ones
Molecular Vibrations:
The frequency decreases with....
increasing atomic weight
Molecular Vibrations:
The frequency increases with....
bond energy
-stronger bonds usually vibrate faster than weaker bonds (assuming the atoms have similar masses)
What is the infrared spectrum?
-a graph of energy absorbed by a molecule as a function of the frequency or wavelength of light
-the simple stretching vibrations in the 1600 to 3500 cm-1 region are the most characteristic and predictable
-the region of the IR spectrum containing most of these complex vibrations (600 to 1400 cm-1) is commonly called the fingerprint
-stronger polar bonds (C=O) may absorb so strongly that they also produce overtone
What does the term overtone mean?
relatively small peaks at a multiple (usually double) of the fundamental vibration frequency
T or F: All molecular vibrations absorb infrared radiation.
false
How do you determine whether a compound absorbs infrared radiation?
-we need to consider how an electromagnetic field interacts with a molecular bond
-the key to this interaction lies with the polarity of the bond, measured as its dipole moment
When the electric field is in the same direction as the dipole moment, the bond is compressed, and its dipole moment __________________.
decreases
When the field is opposite the dipole moment, the bond stretches, and its dipole moment ___________.
increases
Vibrations of bonds with dipole moments generally result in IR absorptions and are said to be _______________.
IR-Active
When is a vibration said to be IR-inactive?
-if the bond is symmetrical and has zero dipole moment, the electric field does not interact with the bond
-because the vibration produces no change in the dipole moment, there is no absorption of energy
Infrared spectra can be measured using what things?
liquid, solid, or gaseous samples
What does the infrared spectrometer do?
measures the frequencies of infrared light by a compound
Summary of IR Stretching Frequencies:
Which functional groups are found around the frequency 3300 cm-1?
1. Alcohol: O-H (always broad)
2. Amine, amide: N-H (may be broad, sharp, or broad with spikes)
3. Alkyne: C(triple bond)C-H (always sharp, usually strong)
Summary of IR Stretching Frequencies:
Which functional groups are found around the frequency 3000 cm-1?
1. Alkane: C-H (just below the 3000 cm-1)
2. Alkene: C=C-H (just above the 3000 cm-1)
Summary of IR Stretching Frequencies:
Which functional groups are found around the frequency 2200 cm-1?
1. Alkyne: -C(triple bond)C- (just below the 2200 cm-1)
2. Nitrile: -C(triple bond)N (just above the 2200 cm-1)
Summary of IR Stretching Frequencies:
Which functional groups are found around the frequency 1710 cm-1?
1. Carbonyl: -C=O
-amides are lower about 1650 cm-1 (conjugation lowers frequency)
-Ketones and acids just about 1710 cm-1
-aldehydes about 1725 cm-1
-esters higher about 1735 cm-1
Summary of IR Stretching Frequencies:
Which functional groups are found around the frequency 1660 cm-1?
1. Alkene: C=C (conjugation lowers frequency aromatic C=C about 1600)
2. Imine: C=N (stronger than C=C)
3. Amide: C=O (stronger than C=C)
What is the fingerprint region?
1400-600 cm-1
Carbon-Carbon bond stretching frequencies:
C-C bonds are found around which range?
1200 cm-1
Carbon-Carbon bond stretching frequencies:
C=C bonds are found around which range?
1660 cm-1
Carbon-Carbon bond stretching frequencies:
C(triple bond)C bonds are found around which range?
<2200 cm-1
Infrared Spectrometry of Hydrocarbons:
What are the characteristics of C=C bonds?
1. Absorption intensity: weak to medium
2. Region: 1600 to 1680 cm-1
Infrared Spectrometry of Hydrocarbons:
Characteristic C=C stretching frequencies:
1. Isolated C=C: 1640-1680 cm-1
2. Conjugated C=C: 1620-1640 cm-1
3. Aromatic C=C: approx. 1600 cm-1
Infrared Spectrometry of Hydrocarbons:
What are the characteristics of C(triple bond)C bonds?
1. Absorption intensity: weak to medium
-terminal alkynes C(triple bond)C-H usually give sharp stretching signals of moderate intensity
-internal alkynes R-C(triple bond)C-R usually give a weak or absent stretching absorption (due to small dipole moment)
2. Region: 2100 to 2200 cm-1
Carbon-Hydrogen Bond Stretching:
What is the characteristic C-H stretching frequency of an Alkane?
-An Alkane, which is sp3 hybridized, has a one-fourth s character (meaning stronger and stiffer bonds)
-region: 2800-3000 cm-1
Carbon-Hydrogen Bond Stretching:
What is the characteristic C-H stretching frequency of an Alkene?
-an alkene is sp2 hybridized, so it has a one-third s character (meaning stronger and stiffer bonds)
-region: 3000-3100 cm-1
Carbon-Hydrogen Bond Stretching:
What is the characteristic C-H stretching frequency of an Alkyne?
-an alkyne is sp hybridized, so it has one-half s character (meaning stronger and stiffer bonds)
-region: 3300 cm-1 (sharp)
Characteristic Absorptions of Alcohols and Amines:
What region do alcohol O-H groups appear on the mass spectrometry?
3300 cm-1 (broad and strong); looks like a tongue
Characteristic Absorptions of Alcohols and Amines:
What region do acid O-H groups appear on the mass spectrometry?
3000 cm-1 (broad)
Characteristic Absorptions of Alcohols and Amines:
What region do amine N-H groups appear on the mass spectrometry?
3300 cm-1 (broad with spikes)
Characteristic Absorption of Carbonyl compounds:
What is the region frequency for a ketone?
1710 cm-1
Characteristic Absorption of Carbonyl compounds:
What is the region frequency for a aldehyde?
1. C=O stretch will be at 1725 cm-1
2. C-H stretch at 2700,2800 cm-1
Characteristic Absorption of Carbonyl compounds:
What is the region frequency for an acid?
1. C=O stretch at 1710 cm-1
2. O-H stretch at 2500-3500 cm-1
Characteristic Absorptions of C-N bonds:
What are the characteristics of these types of bonds?
1. Absorption intensity: medium to strong
2. C-N stretches: 1200 cm-1
3. C=N stretches: 1660 cm-1
4. C(triple bond)N stretch: >2200 cm-1
An infrared spectrum is valuable in three ways:
1. It indicates the functional groups in the compound
2. It shows the absence of other functional groups that would give strong absorption if they were present
3. It can confirm the identity of a compound by comparison with a known sample
Summary of IR Stretching Frequencies:
Ethers, esters, and alcohols also show C-O stretching between what frequencies?
1000 and 1200 cm-1
Aromatic overtones and combination bands appear in what frequency region?
1650-2000 cm-1