chemistry topic 2 (copy)
0.0(0)
Card Sorting
1/168
Last updated 4:43 PM on 3/3/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
169 Terms
1
New cards
what are ions?
charged particles
2
New cards
why do atoms become ions?
to become stable/ full outer shell
3
New cards
how do metals form ions?
lose electrons to become positive
4
New cards
how do non-metals form ions?
gain electrons to become negative
5
New cards
what atoms react together and why?
non-metal and metal because they have opposite charges, to become stable
6
New cards
what happens when atoms react (ionic bonding)?
they become strongly attracted to eachother by electrostatic forces (called ionic bonding)
7
New cards
what do dot and cross diagrams show?
the arrangement of electrons in an atom or ion. each electron is represented by a dot or cross
8
New cards
when do you use dot and cross diagrams?
for ionic bonding (non metal + metal)
9
New cards
what are disadvantages of dot and cross diagrams? (3)
doesn’t show the structure of the compound, the size of ions, or how they’re arranged
10
New cards
what are ionic compounds?
compounds that only contain ionic bonds
11
New cards
what is ionic bonding?
the strong electrostatic forces of attraction between oppositely charged metal and non-metal ions
12
New cards
what force is in ionic bonding?
(very strong) electrostatic forces of attraction
13
New cards
what structure are ionic compounds?
giant ionic lattice
14
New cards
what is a giant ionic lattice?
large number of ions regularly arranged in a repeating pattern, repeated in all directions making a giant 3D lattice structure
15
New cards
what is table salt as an element?
sodium chloride
16
New cards
describe advantages of a ball and stick model (3)
shows the regular pattern of an ionic crystal, how the ions are arranged and suggests that the crystal extends beyond whats shown in the diagram
17
New cards
describe disadvantages of a ball and stick model (2)
isn’t to scale so relative sizes of the ions may not be shown, and in reality there aren’t gaps between ions
18
New cards
describe advantages of model 2 of ionic compounds (2)
shows relative sizes of the ions and the regular pattern of an ionic crystal
19
New cards
describe a disadvantage of model 2 of ionic compounds (1)
only lets you see the outer layer of the compound
20
New cards
what are the melting and boiling points of ionic compounds like?
in giant ionic lattices, they’re high because it requires large amounts of energy to overcome the many strong electrostatic forces of attraction
21
New cards
can ionic compounds conduct electricity?
they can only conduct when molten (liquid) or dissolved in a solution, as ions (charged particles) are present, free to move and carry a charge throughout the circuit
22
New cards
what happens when non metal atoms bond together?
they share pairs of electrons to make covalent bonds
23
New cards
what is a covalent bond?
when atoms share electrons with each other so they both have full outer shells (to become stable)
24
New cards
how is the positive nuclei (of the bonded atoms) attracted to the shared pair of electrons?
by electrostatic forces, making covalent bonds very strong
25
New cards
why do atoms only share electrons in their outer shells?
they have the highest energy levels
26
New cards
where does covalent bonding happen?
in 2 non metals
27
New cards
what diagram do you use to show covalent bonds?
dot and cross diagrams
28
New cards
why are dot and cross diagrams useful for covalent bonds? (1)
they show which atoms the electrons in a covalent bond come from
29
New cards
why are dot and cross diagrams not useful for covalent bonds? (2)
doesn’t show relative sizes of atoms, or how they are arranged in the space
30
New cards
what are advantages of stick diagrams? (1)
show how atoms are connected in large molecules
31
New cards
what are disadvantages of stick models? (2)
don’t show 3D structure of molecules, or which atoms the electrons in the covalent bond have come from
32
New cards
what are advantages of 3d models? (2)
shows the atoms and the covalent bonds, and their arrangement in the space next to each other
33
New cards
what are disadvantages of 3d models? (2)
they can be confusing because there are lots of atoms to include, and doesn’t show where the electrons in the bonds have come from
34
New cards
what are simple molecular substances?
made up of molecules containing a few atoms joined together by covalent bonds
35
New cards
what do substances containing covalent bonds usually have?
simple molecular substances
36
New cards
what are the atoms between the molecules (in covalent bonds) held together by?
very strong covalent bonds
37
New cards
in covalent bonds, how weak/ strong are the the forces of attraction between the molecules
VERY weak
38
New cards
why do covalent bonds have low melting/boiling points?
they’re simple molecules, so the weak forces of attraction between covalent molecules in a liquid phase (intermolecular forces) can be overcome with little heat energy
39
New cards
what state of matter are most molecular substances at room temp?
gases or liquids
40
New cards
what happens as molecules get bigger (in simple molecular bonds)
the strength of intermolecular forces increases, so more energy is required to break them, so melting and boiling points increase
41
New cards
do molecular compounds conduct electricity?
no, because they aren’t charged, so there are no free electrons or ions
42
New cards
how are giant covalent structures bonded together?
strong covalent bonds
43
New cards
are the melting and boiling points high in giant covalent structures and why?
yes, very high, as lots of energy is required to break the covalent bonds between the atoms
44
New cards
why don’t the atoms in giant covalent structures conduct electricity?
dont contain charged particles
45
New cards
describe the physical appearance of diamonds
each carbon atom forms 4 covalent bonds in a very rigid giant covalent structure
46
New cards
\
describe the physical appearance of graphite
describe the physical appearance of graphite
each carbon atom forms 3 covalent bonds, to create layers of hexagons. each carbon atom also has 1 delocalised electron
47
New cards
describe the physical appearance of silicon dioxide
this is what sand is made of. each grain of sand is 1 giant structure of silicon and oxygen
48
New cards
what is an allotrope?
different structural forms of the same element in the same physical appearance
49
New cards
why are diamonds really hard? ;)
because it has a giant covalent structure, made up of carbon atoms that each form 4 covalent bonds. (Rigid tetrahedral shape)
50
New cards
why does diamonds have very high melting/ boiling points?
there are many strong covalent bonds between every carbon atom
51
New cards
does diamond conduct electricity?
no, there are no charged particles (no free electrons)
52
New cards
why is graphite an ideal lubricating material?
there aren’t many covalent bonds between layers, they’re held together weakly. This means they’re free to move over each other, so it is soft and slippery
53
New cards
is graphite’s melting point high or low, and why?
high, because the covalent bonds in the layers need loads of energy to break them
54
New cards
how many of carbons outer electrons are used in bonds?
3
55
New cards
how does the amount of outer electrons affect graphites properties?
it means each carbon atom has 1 delocalised electron, and can move, so graphite conducts electricity and thermal energy
56
New cards
what is graphene?
a sheet of carbon atoms joined together in hexagons
57
New cards
how thick is the sheet of carbon atoms in graphene?
one atom thick, making it 2D
58
New cards
is graphene strong or not?
it’s very strong, as there is a network of covalent bonds
59
New cards
why is graphene used to make composite materials stronger?
it’s incredibly light, so will improve their strength without adding much weight
60
New cards
can graphene conduct electricity and why?
yes, it has delocalised electrons so it can conduct electricity through the whole structure
61
New cards
what substances contain the same atoms (carbon)?
diamond, graphite, graphene
62
New cards
what are fullerenes?
molecules of carbon, shaped like closed tubes or hollow balls
63
New cards
what was the first fullerene to be discovered?
Buckminsterfullerene
64
New cards
what is the molecular formula of Buckminsterfullerene?
C(60)
65
New cards
what does the carbon atoms in buckminsterfullerene form?
a hollow sphere containing 20 hexagons and 12 pentagons
66
New cards
what can fullerenes be used to do?
to cage other molecules
67
New cards
what does the fullerene structure form around?
another atom or molecule, which is trapped inside the fullerene. This can be used for delivering drugs around the body
68
New cards
what can fullerenes be used for? (4)
delivering drugs around the body, catalysts, (spherical fullerenes for) lubricants, (cylindrical fullerenes for) strengthening materials
69
New cards
what is a benefit of fullerenes large surface areas'?
they can help make great industrial catalysts. Individual catalyst molecules could be attached to the fullerenes
70
New cards
what can fullerenes form?
nanotubes
71
New cards
what are nanotubes?
tiny carbon cylinders
72
New cards
what is the ratio between the length and the diameter of nanotubes?
very high
73
New cards
why can nanotubes conduct electricity and thermal energy?
delocalised electrons can move throughout the structure
74
New cards
why do nanotubes have a high tensile strength (strong)?
because of high length to diameter ratio, many strong covalent bonds
75
New cards
why do nanotubes have a high melting point?
because each atom is covalently bonded to 3 others
76
New cards
what is the technology that uses very small particles like nanotubes?
nanotechnology
77
New cards
what can nanotubes be used in?
in electronics, or to strengthen materials without adding much weight
78
New cards
what are most polymers held together by?
covalent bonds
79
New cards
what happens in a polymer?
lots of small units are linked together to form a long molecule that has repeating sections.
80
New cards
what is the short section you can draw out for a polymer instead of the whole molecule?
the repeating unit
81
New cards
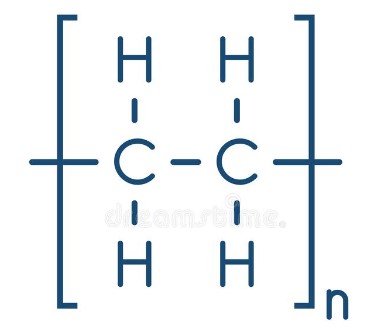
what is this polymer called?
poly(ethene)
82
New cards
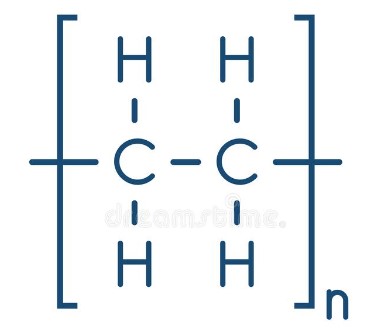
what is the bit in the brackets?
the repeating unit
83
New cards
what does the bonds through the bracket join up to?
the next repeating unit
84
New cards
what is the n in the polymer?
the symbol that tells you the unit is repeated lots of times
85
New cards
how do you find the molecular formula of a polymer? (make an example for poly(ethene))
write down the molecular formula of the repeating unit in brackets, and put an ‘n’ outside. eg. poly(ethene) = (C2H4)n
86
New cards
\
are the intermolecular forces between polymer molecules smaller or larger than simple covalent molecules?
are the intermolecular forces between polymer molecules smaller or larger than simple covalent molecules?
larger than between simple covalent molecules, so more energy is needed to break them
87
New cards
what state are most polymers?
solid (s)
88
New cards
are the intermolecular forces between polymer molecules smaller or larger than ionic or covalent bonds?
smaller and weaker, so they generally have lower boiling points than ionic or giant molecular compounds
89
New cards
what do metals consist of?
a giant structure
90
New cards
what electrons in metals are delocalised?
the electrons in the outer shell
91
New cards
what force is between the positive metal ions and the shared negative electrons?
strong electrostatic forces of attraction
92
New cards
what do the forces of attraction in metals do?
they hold the atoms together in a regular structure and are known as metallic bonding
93
New cards
is metallic bonding strong or weak?
strong
94
New cards
what does the substances held together by metallic bonding include?
metallic elements and alloys
95
New cards
what produces all the properties of metals in metallic bonds?
delocalised electrons
96
New cards
is the electrostatic forces between the metal atoms and the delocalised sea of electrons strong or weak?
strong, so they need lots of energy to be broken
97
New cards
what does the strong electrostatic forces in a metal mean?
most compounds with metallic bonds have very high melting and boiling points, so they are generally solid at room temperature
98
New cards
why are metals good conductors of heat and electricity?
they carry electrical charge and thermal energy through the whole structure, so they are good conductors of electricity and heat
99
New cards
what can the layers of atoms in a metal do?
they can slide over each other, making metals malleable, which means that they can be bent, hammered or rolled into flat sheets
100
New cards
why arent pure metals good for certain metallic bonding jobs?
because they are often too soft when they are pure, so they’re mixed with other elements to make them harder ;)