Science 10-Half Life Assignment
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
Half-life
The time required for half of a sample of a radioactive substance to decay or transform into a different substance. It is a key concept in nuclear chemistry and radioactive dating.
How long is one half-life of carbon-14?
Approximately 5,730 years.
What is the percentage of carbon-14 after one, two, and three half-lives?
50% after 1 half-life, 25% after 2, and 12.5% after 3
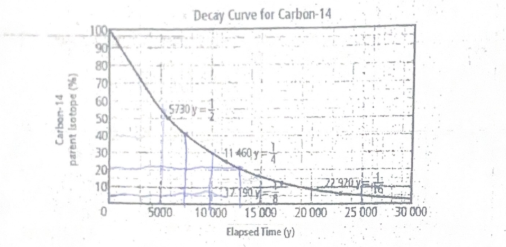
Estimate the percentage carbon-14 after 5000 years, 10000 years, and 15000 years
Approximately 87%, 75%, and 62.5%
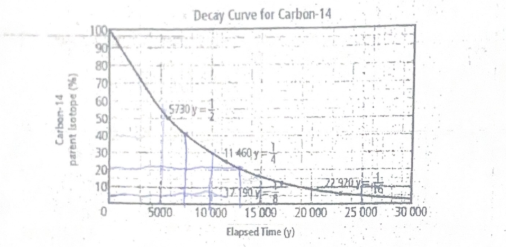
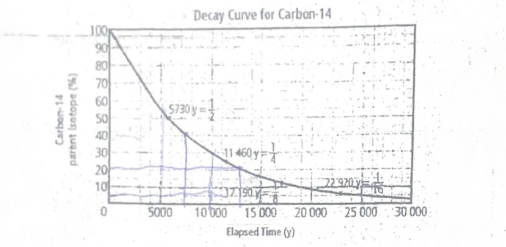
Use the graph to estimate number of years based since the organism died if the percentage of parent isotope that remains is 40%, 20%, 5%
Approximately 8,000 years for 40%, 14,000 years for 20%, and 22,000 years for 5%.
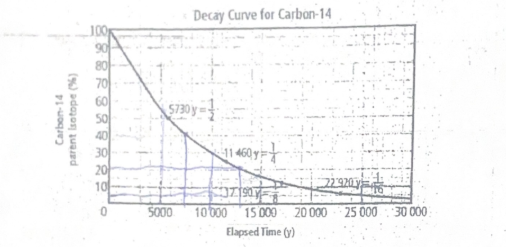
Why is carbon-14 half-life measurements are not effective in dating an organism that has been dead for more than 50,000 years
Because after this time, the amount of carbon-14 remaining is so minimal that it becomes difficult to accurately measure, leading to unreliable dating results.