11) Carbonate solubility
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
explain the calcium carbonate pH example
K1 = K1 = [H+]*[HCO3-] / [H2CO3]
K1 / [H+] = [HCO3-] / [H2CO3]
K1 / 10^-pH = [HCO3-] / [H2CO3]
K2 = [H+]*[CO32-] / [HCO3-]
K2 / [H+] = [CO32-] / [HCO3-]
K2 / 10^-pH = [CO32-] / [HCO3-]
If pH=6.35, activities of HCO3- and H2CO3 are equal
If pH>6.35, ratio for K1 is higher, meaning more bicarbonate, low pH means more H+ ions, pushing the reaction to the left (more bicarbonate)
If pH<6.35, ratio for K1 will be lower, meaning more carbonic acid
is seawater saturated with CaCO3
what is saturation horizon
we know for the reaction, K=Ca*CO32- / CaCO3
IAP > Ksp, so seawater is saturated with CaCO3
deepest depths have little to no CaCO3 in the bottom sediments
carbonate producing orgs are active in near-surface ocean water
shells dissolve at 5km
saturation horizon: carbonate compensation depth, no carbonates are preserved in sediments below this depth
conservative element vs nonconservative elements
conservative: abundance in seawater does not change that much with depth, remains constant, ratio of one conservative element to another remains constant
examples: Ca, K, Na, Cl
nonconservative: abundance in seawater changes with depth, vary because they participate in biological reactions, nutrients, formation of org compounds, vary depending on how much they are used up
examples: carbonate
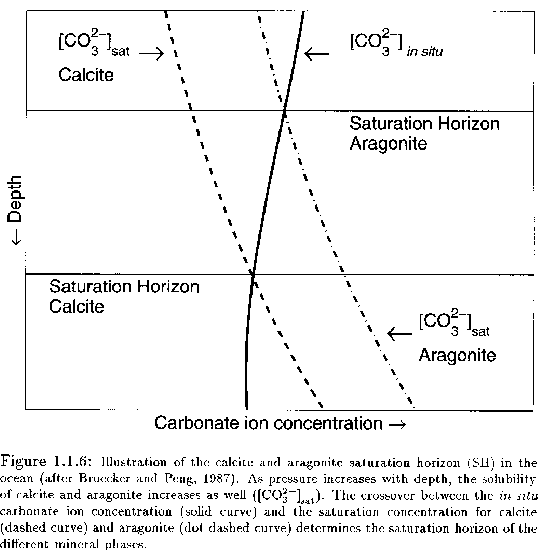
explain this graph
aragonite is more soluble, first curve is carbonate needed to form calcite, second is for aragonite
solid line represents actual conc of carbonate in the ocean
first horizontal line represents saturation line for aragonite: above it aragonite is stable (saturated), below it aragonite dissolves
second horizontal line represents saturation line for calcite: above it calcite is stable, below it calcite dissolves. lysocline
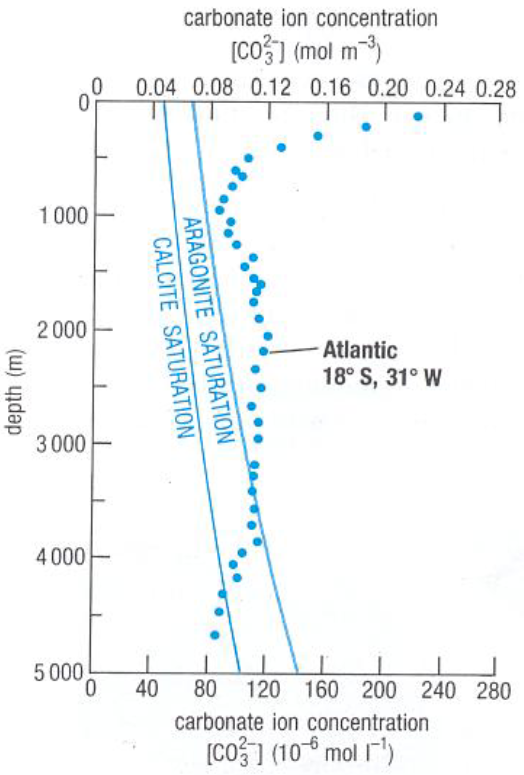
explain this graph
dotted line is how carbonate concs vary in oceans
at 3200m is where aragonite saturation level is reached
at 4500m is where calcite saturation level is reached, where any sediments accumulated there will not have any carbonates preserved
why does solubility of CaCO3 increase with depth?
temp: decreases, so Ksp for CaCO3 increases. Boiling water precipitates calcite because its less soluble in warm water, this is why carbonate production occurs in warm tropical waters. solution of carbonates is exothermic
pressure: increases, so Ksp for CaCO3 increases. With calcite+water vs ions in water, the ions occupy a smaller volume, so when you increase pressure, it’s thermodynamically favourable for ions to exist
more CO2 dissolved: increases solubility of carbonates. ALso increased pressure means higher dissolved CO2)
what is the effect of dissolved CO2
when it combines with water it produces carbonic acid, which turns into bicarbonate, then carbonate, each time with aciditity (H+ ions)
CaCO3 + H2O +CO2 → 2HCO3- + Ca2+
increased CO2 drives reaction to the right, dissolving calcite
higher CO2 in atmosphere means
higher CO2 in oceans: 1/3 of CO2 in atmosphere goes into oceans
less carbonate ions, more carbonic acid, more free H+ ions, more bicarbonate, fewer smaller marine calcifiers