exothermic and endothermic reactions
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
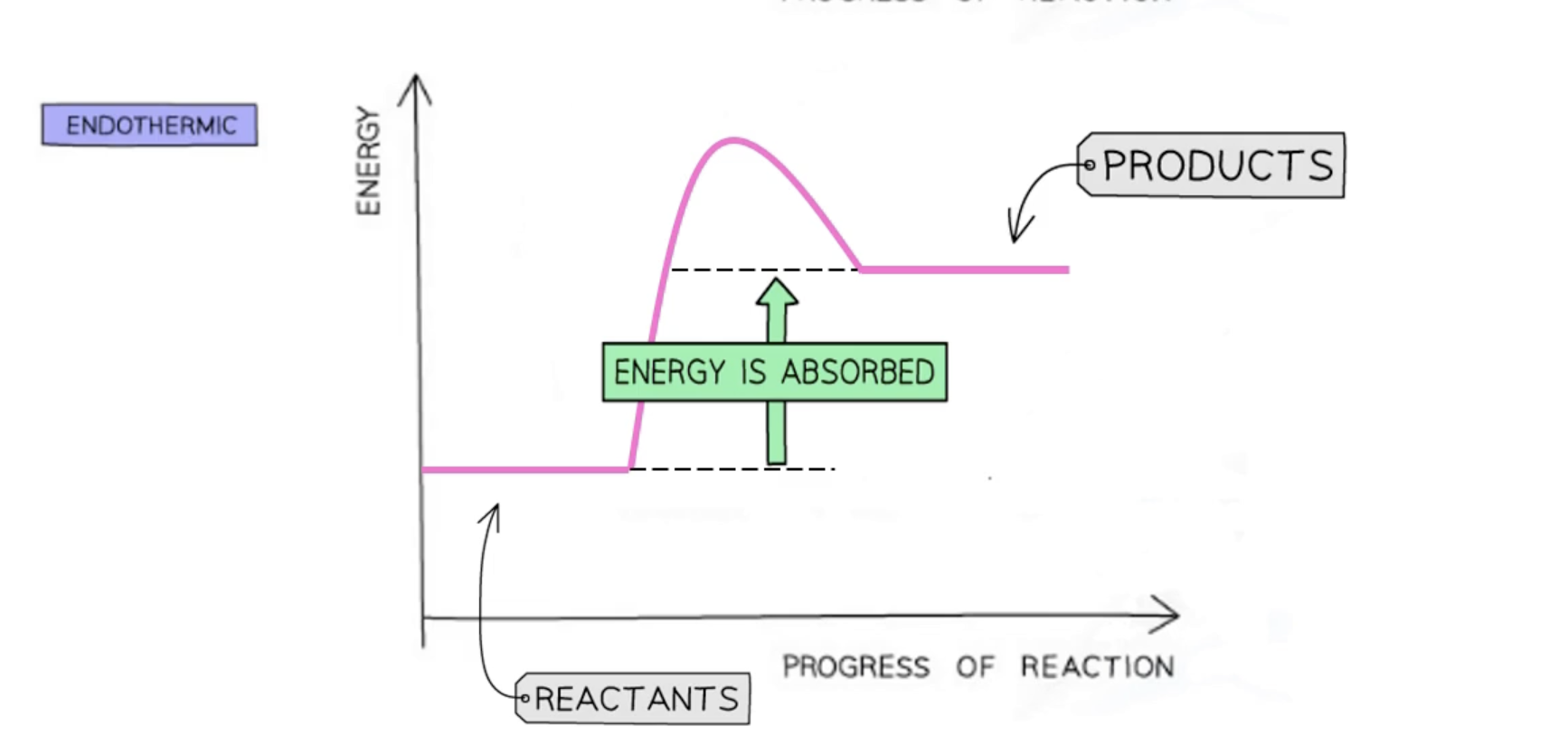
endothermic reaction pathway diagram
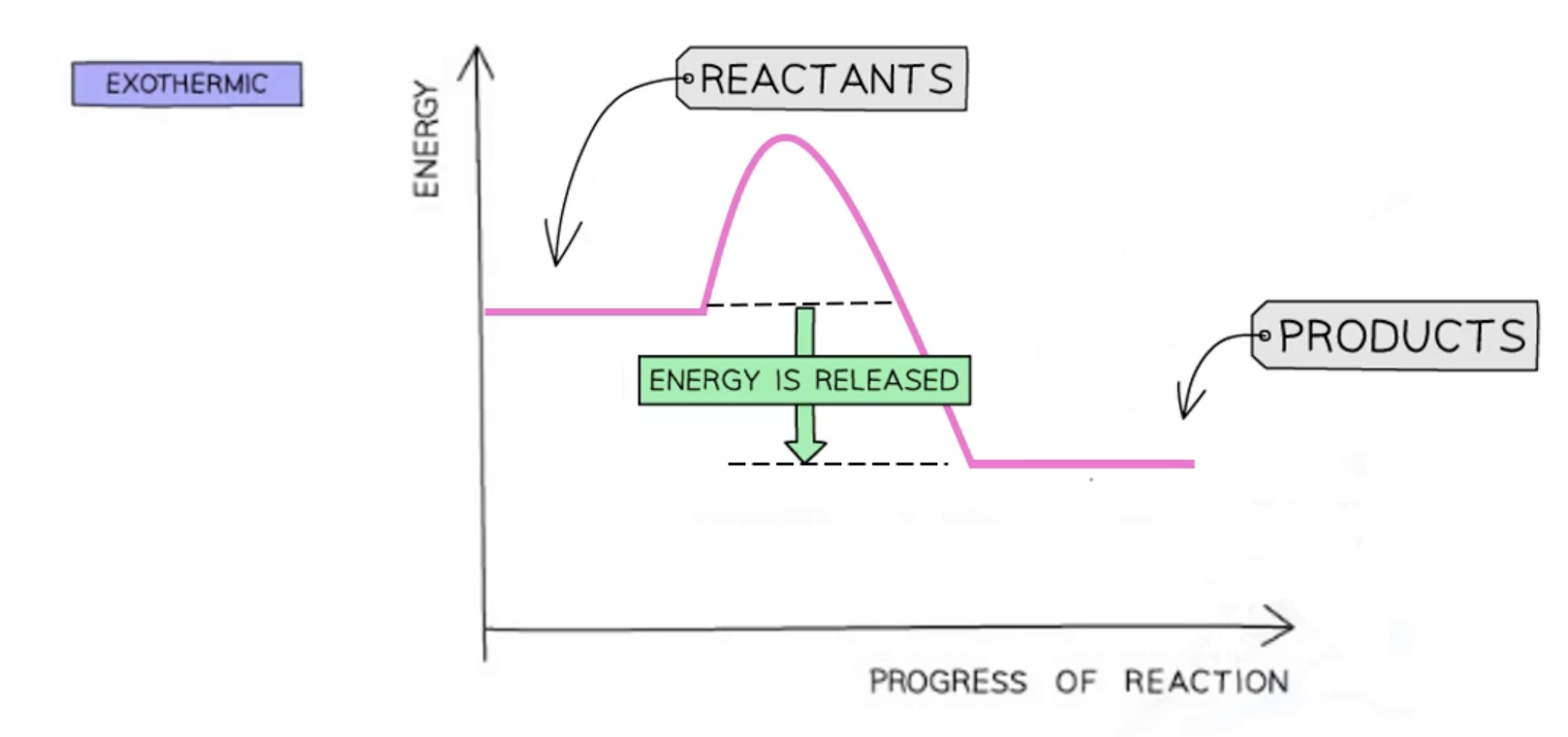
exothermic reaction pathway diagram
in endothermic reactions
thermal energy is taken during a reaction
energy is taken in from the surrounding
more energy is absorbed than released
temperature of the surrounding decreases
thermal decomposition and electrolysis
energy of the products will be higher than the energy of the reactants
overall energy change is positive
endothermic → entering
in exothermic reactions
thermal energy is given out during a reaction
energy is given out to the surroundings
more energy is released than absorbed
temperature of the surroundings increase
combustion and neutralisation reactions
the energy of the products will be lower than the energy of the reactants
overall energy change is negative
exothermic → exiting
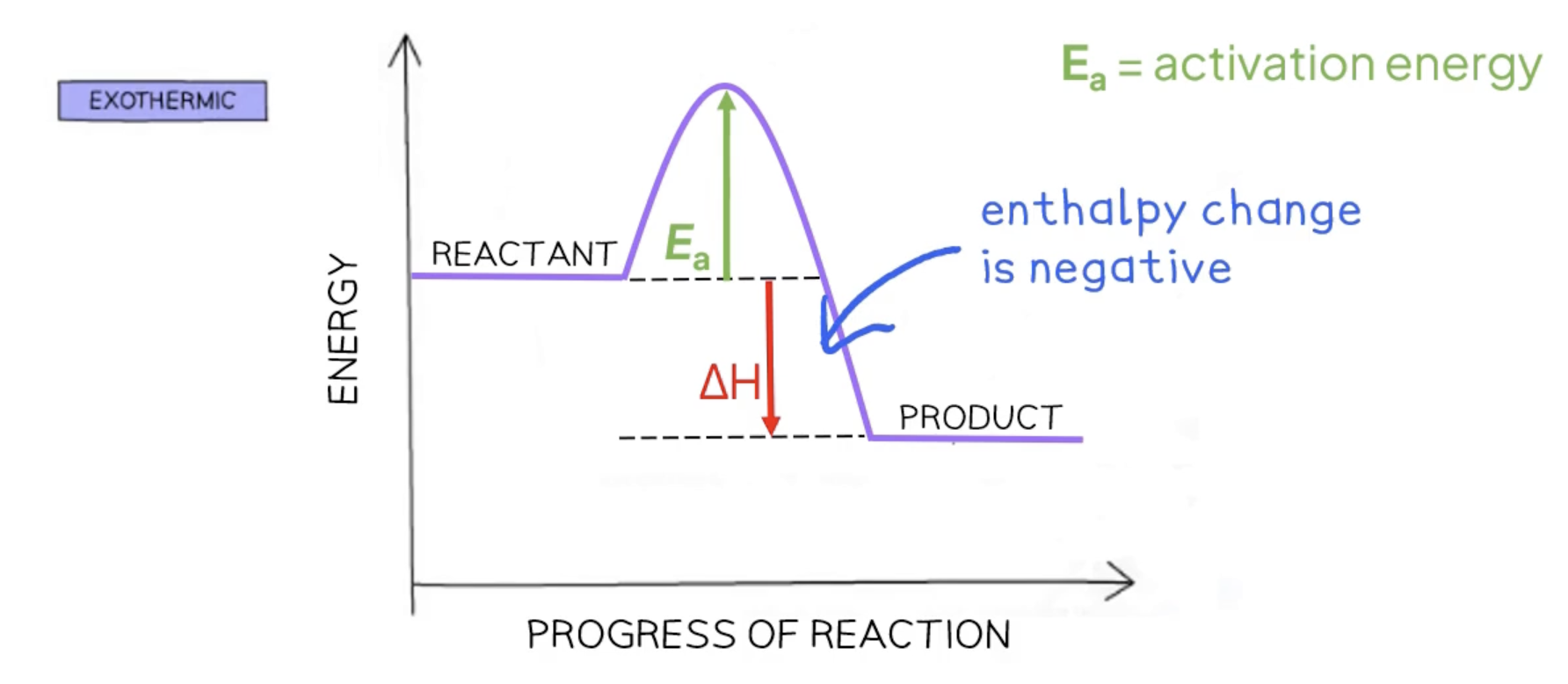
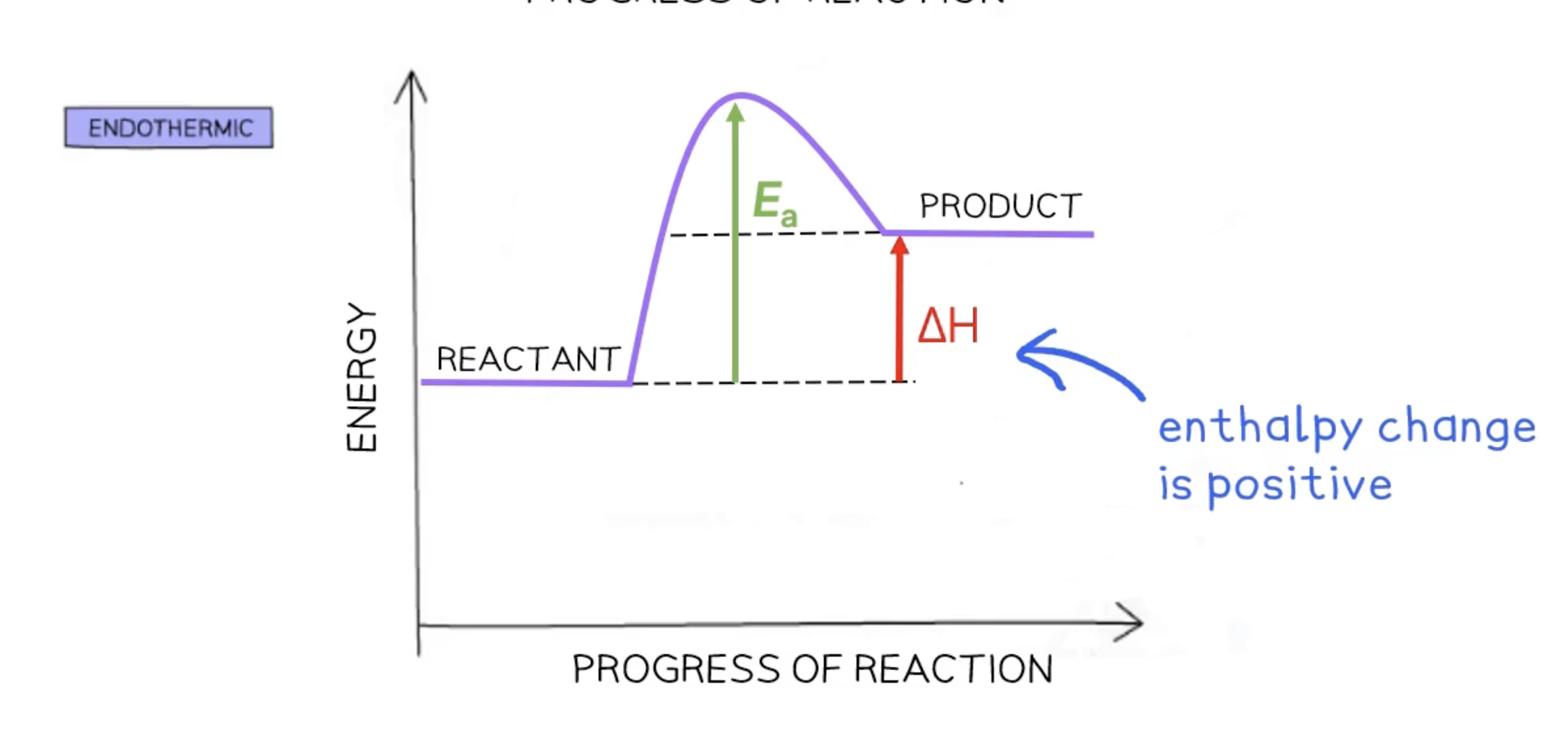
activation energy Ea
the minimum amount of energy colliding particles must have to react
enthalpy change ΔH
refers to the transfer of thermal energy during a reaction
exothermic reactions - enthalpy is negative
endothermic reaction - enthalpy is positive
reaction
to have a product formed, particles need to collide with activation energy which allows a reaction to occur and form a product
bond breaking
endothermic reaction
bond making
exothermic reaction
energy change formula
