exam 2 bio104
1/58
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
59 Terms
integral proteins
extend part-way or all the way through the plasma membrane
transmembrane proteins
type of integral protein that goes across the membrane, involved in transport.
types of membrane proteins
channel, carrier, cell recognition, receptor, enzymatic
channel protein
provide passageways for molecules
carrier protein
bind specific substances and change shape to transport them
cell recognition protein
glycoprotein that helps the body defend itself against pathogens
receptor protein
a protein that binds specific signal molecules, which causes the cell to respond
enzymatic protein
protein that catalyzes a specific reaction
explain why the cell membrane is considered a fluid structure
the phospholipid bilayer allows lipids and proteins to move laterally, giving it its flexible nature
fluid mosaic model
model that describes the arrangement and movement of the molecules that make up a cell membrane
explain what occurs with high temperature in the fluid mosaic model
increased fluidity
explain what occurs with low temperature in the fluid mosaic model
decreased fluidity
cholesterol in fluid mosaic model
keeps membrane fluid by preventing tight packing at low temps and reducing excessive movement at high temps
passive transport
requires NO energy, movement of molecules from high to low concentration, moves with the concentration gradient
simple diffusion
molecules pass directly through the membrane without assistance
facilitated diffusion
molecules move across the membrane with help of carrier proteins
osmosis
diffusion of water through aquaporins or directly across the membrane.
hypertonic solution
water moves out of the cell causing it to shrink
hypotonic solution
water moves into the cell causing it to swell
isotonic cell
cell is stable, water movement is balanced
active transport
requires energy, moves substances against their concentration gradient
Na+/K+ pump
uses ATP to move 3 Na+ out and 2 K+ ions in the cell, gradient maintains resting membrane potential
cotransporters
uses an existing ion gradient (from active transport) to move another substance.
ex. Na+/glucose cotransporters in kidneys and small intestine - bring glucose into the cells
exocytosis
a process by which the contents of a cell vacuole are released to the exterior through fusion of the vacuole membrane with the cell membrane
endocytosis
process by which a cell takes material into the cell by infolding of the cell membrane
phagocytosis
type of endocytosis in which a cell "eats" large particles or whole cells
pinocytosis
type of endocytosis in which the cell "drinks" extracellular fluid and its dissolved solutes
kinetic energy
energy of motion
ex. running person
potential energy
stored energy
ex. battery
entropy
a measure of disorder where heat is generated
ex. ice melting into water due to molecules becoming disordered
first law of thermodynamics
energy cannot be created or destroyed, instead changed from one form to another
ex. turning on a lightbulb changes electrical energy -> light energy
second law of thermodyanmics
energy cannot be changed from one form to another without the loss of useable energy
ex. heat lost from a engine during combustion
ATP (adenosine triphosphate)
consists of adenine + ribose (sugar) + 3 phosphate groups
- ATP loses a phosphate to become ADP and releases energy
- ADP gains a phosphate to become ATP and stores energy
ATP in cellular work
ATP binds myosin head and 1 phosphate is broken, providing energy for muscle contraction, allows myosin head to reach up and connect with actin to make the contraction happen
exergonic reaction
break bonds between molecules and releases heat
ex. cellular respiration
endergonic reaction
bonds are made and energy stored in bonds (requires energy)
ex. photosynthesis
coupling
exergonic reactions (like ATP hydrolysis) drive endergonic reactions (like protein synthesis)
explain how enzymes lower the activation energy to speed up a reaction
enzymes lower activation energy, making reactions happen faster by stabilizing the transition state
enzyme reaction
enzymes bind specific substrates at the active site, forming the enzyme-substrate complex, which is then converted into the product
explain why enzymes are usually only specific to 1 substrate
specific to one substrate due to the shape of the active site
enzyme degradation
enzyme breaks down a molecule into smaller parts
enzyme synthesis
enzyme builds a larger molecule from smaller parts
factors that affect enzyme activity
substrate concentration: higher concentration increases activity to a certain point
temperature: too high=denatures enzyme
too low=slows enzyme activity
pH: extreme levels (basic/acidic) can denature enzyme
explain where the active site and non-competitive inhibition site are on an enzyme and how they work when bound by a molecule
active site: where substrate binds and undergoes chemical reaction
noncompetitive inhibition: inhibitor molecule binds enzyme somewhere other than the active site
explain enzyme co-factor
non-proteins needed for enzyme to work/catalyze a chemical reaction
ex. vitamins (iron, copper,zinc) and coenzymes (FAD, NAD+, NADP+)
explain why cellular respiration occurs without oxygen (anaerobic respiration)
without oxygen, fermentation is used to regenerate NAD+ for glycolysis - produces 2-4ATP
aerobic respiration (O2 needed)
1. glycolysis (cytoplasm): breakdown of glucose into 2 pyruvate
2. preparatory phase (mitochondria): 2 pyruvate -> 2 acetylCoa
3. Krebs cycle (mitochondria): generate electron carriers (NADH, FADH2, and ATP) - occurs twice per glucose
4. ETC + chemiosmois (mitochondria): electrons from NADH and FADH2 are used to create proton gradient which drives ATP production
chemiosmosis
a process for synthesizing ATP using the energy of an electrochemical gradient and the ATP synthase enzyme.
energy carrier molecules
molecules that transport energy in the form of high-energy electrons
ex. ATP, NADH, FADH2
anaerobic respiration (fermentation)
occurs without oxygen and allows glycolysis to continue with NAD+
types of fermentation
alcohol: yeast cells - 2 ATP, 2 alcohol, 2CO2, 2ADP
lactic acid: animal cells - 2 ATP, 2 lactate, 2 ADP
explain why fermentation produces less ATP than aerobic
fermentation does not use Krebs cycle or ETC, where most ATP would be generated
anaerobic - 2-4 ATP
aerobic - 36-38 ATP
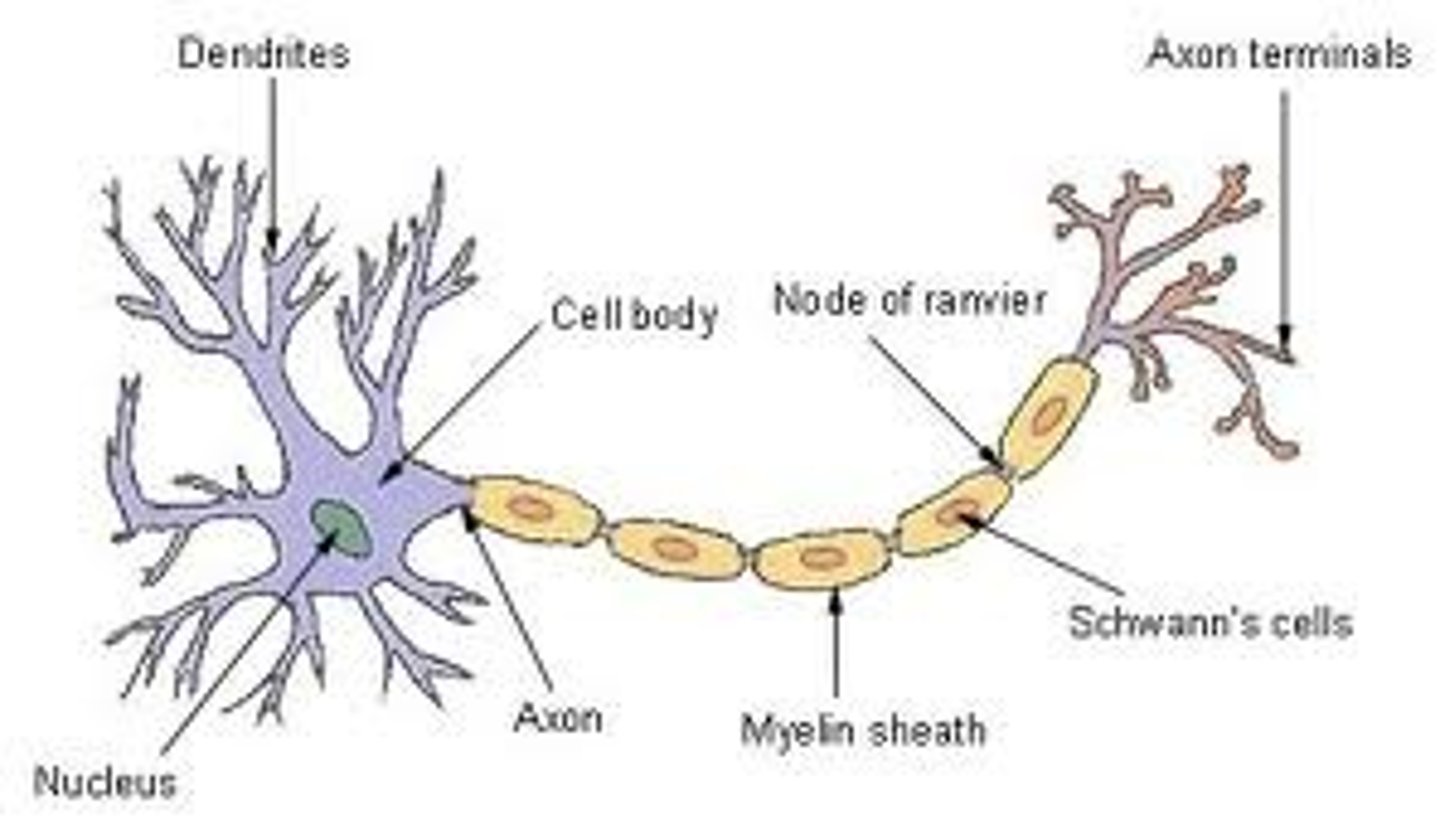
structure of neuron
synaptic terminals: transmit signals from other neurons
dendrites: receive signals from other neurons
cell body: integrates signals; coordinates neurons metabolic activities
axon: conducts the action potential
myelin sheath: speeds up signal transmission
Central Nervous System vs Peripheral Nervous System
CNS: Brain and spinal cord
PNS: nerves radiating out from spinal cord to rest of body - Somatic (controls voluntary muscle movement) and Autonomic (controls involuntary functions)
classes of neurons
sensory (afferent): respond to a stimulus, motor (efferent): activate muscles and glands to respond to stimuli, interneurons: process information and connect sensory and motor neurons.
membrane voltage (mV)
resting potential (-70mV) = Na+(outside) high and K+ (inside) high
threshold (-55mV) = Na+(outside) high and K+ (inside) high
action potenital (+35mV) = Na+ rushes in large and K+ (inside)
repolarization to resting (-70mV) = Na+ inside is pumped out and K+ exiting is pumped back in
resting potenial
transports 3 Na+ out, 2 K+ in, maintaining a negative charge inside.
K+ leak channels allow some K+ to move out, making inside more negative.
depolarization
voltage-gated Na+ channels open, causing Na+ to rush in and the inside to become more positive.
repolarization
Voltage-gated K+ channels open, K+ exits, making the inside negative again.
Na+ channels close to prevent more Na+ from entering.