HMB300 Midterm 1 Review
1/99
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
100 Terms
Characteristics of neurological diseases
Progressive neurological symptoms
Selective loss of neurons (SNc and basal ganglia)
Presence of protein aggregates (beta amyloid, alpha-synuclein)
No curative treatments
What is the second most common neurodegenerative disease
Parkinson’s disease
Symptoms of Parkinson’s disease
Muscle rigidity (unsmooth movements)
Bradykinesia (slow movements)
Impaired gait (shuffling movement)
What are the multiple factors involved in a Parkinson’s Disease with a strong environmental component?
The multiple factors involved in this disorder include environmental exposure, genetic factors, and gene-environment interactions. Genes such as LRRK2, DJ1, PRKN, PINK1, and SNCA, which are responsible for protein folding and maturation, are important to note. Additionally, the aggregation of a-synuclein (aSyn) is thought to be a significant factor, as almost 100% of Parkinson's patients have abnormalities related to a-synuclein.
What are the neurodegenerative disease aetiology of PD?
Dementia with Lewy bodies
Multiple system atrophy
Progressive supranuclear palsy
What are the structural brain lesion aetiology of PD?
Cerebrovascular disease (atherosclerosis) → minimising hardening of arteries in brain
infectious/post-infectious e.g. viral encephalitis
Post-traumatic brain injuries – severe concussions?
What are the drug aetiologies of PD?
Overuse of antipsychotic drugs (particularly phenothiazines, butyrophenones, resperine)
What are the possible environmental cuases of PD?
Higher numbers of PD in areas with high insecticide use
How are toxicology models of PD in mice induced?
Reserpine, MPTP, Methamphetamine, 6-OH-dopamine, Rotenone, and Paraquat.
What are the possible viral causes of Parkinson's Disease?
The events of the 1918 H1N1 outbreak left survivors with von Economo syndrome, which showed Parkinson's Disease markers in the brain. Additionally, the epidemic H5N1 virus was associated with encephalitis lethargica, characterized by slow movement. Animals infected with H5N1 demonstrated acute neurological signs, including mild encephalitis, motor disturbances, and coma. However, there have been no studies examining the longer-term neurological consequences of H5N1 infection among surviving hosts.
What is the most common neurodegenerative disorder, second only to Alzheimer's?
Parkinson's Disease (PD)
What is the approximate percentage of people over 60-65 years old who have Parkinson's disease (PD)?
Approximately 12% of people greater than 60-65 years old have PD.
What is the greatest risk factor for developing Parkinson's disease?
Age is the greatest risk factor for developing Parkinson's disease.
What is the expected increase in the number of people older than 50 years old with Parkinson's disease (PD) by 2030?
The number of people older than 50 years old with PD is expected to increase by more than 50% by 2030.
What is the incidence rate of Parkinson's disease between males and females?
Males have a higher incidence of Parkinson's disease, with a 3:2 ratio of men to women./
What is the experimental model used in the PNAS article?
The experimental model used in the PNAS article is the C57BL/6J mouse, a mouse strain that can be infected by the A/Vietnam/1203/04 H5N1 virus without adaptation.
What happens in the regions infected by the H5N1 virus? (PNAS article)
Activation of microglia, alpha-synuclein phosphorylation, and aggregation occur, persisting long after infection resolution. This leads to the significant loss of dopaminergic neurons 60 days after infection, suggesting a potential link between neurotrophic influenza viruses and CNS disorders characterized by protein aggregation, such as Parkinson's disease.
What were the results of the PNAS article regarding H5N1 in mice?
The results showed that mice affected by H5N1 demonstrated that the virus can travel from any body part to the central nervous system (CNS), leading to the activation of microglia and phosphorylation of alpha-synuclein (a-syn).
What are nucleoproteins (NPs) indicative of within a cell type?
Nucleoproteins (NPs) are a marker for a virus within a cell type. Cells only have NPs after being infected.
What are the staining methods used in the PNAS article?
Iba1 - stains for microglia, DAPI - stains for nucleus, pSER129 Syn - stains for alpha-syn protein aggregation.
Conclusions from the PNAS article
Viral infection can induce a robust induction of cytokines including MCP-1, MIP-1beta, MIP-2, MIP-3alpha, IL-1beta, IL-6, IL-12 (p40), IL-18, and G- CSF, which can be secondarily activated in the brain without direct infection. H5N1 observed in neurons + microglia, but not in astrocyte – virus may enter CNS via cranial nerves (peripheral or enteric)
Endogenous Retroviruses (ERVs)
When sequenced, 8% of human genome contain remnants of ERVs (including Arc, which plays role in memory). Retroviruses can make a DNA copy of their RNA, and insert it into host cell DNA – include HIV, Hepatitis B. In humans, very specialised, resulting from endogenous Borna-like N gene elements that is added to our DNA
What is Borna disease virus (BDV)?
BDV is a neurotropic virus that can infect various species, including all mammals. It is believed to have originated from the domestication of horses. Suggested that many mood disorders and psychoses (i.e. schizophrenia and depression) are caused by remnants of this virus
What is the role of Phosphoprotein 24 (P34) in BDV replication?
Phosphoprotein 24 (P34) is a crucial component of the replication machinery of Borna Disease Virus (BDV). Its mRNA has been found in the blood of patients with mood disorders.
What is the Zika virus (ZIKV) and what are its symptoms?
The Zika virus is a mosquito-borne flavivirus, positive ssRNA virus, that has been circulating in 26 countries and territories. It can cause mild symptoms similar to dengue, such as rash, pain, and conjunctivitis. Some infected individuals may be asymptomatic.
ZIKV, microcephaly and Guillain Barre syndrome
Reports link ZIKV infection to foetal and newborn microcephaly and serious neurological problems (e.g. Guillain-Barre syndrome)
What are the causes of microcephaly?
The causes of microcephaly include chromosomal abnormalities, decreased oxygen to the fetal brain (cerebral anoxia), infection of the fetus during pregnancy (toxoplasmosis, cytomegalovirus, German measles, and chickenpox), exposure to drugs, alcohol, or certain toxic chemicals in the womb, severe malnutrition, and uncontrolled phenylketonuria (PKU) in the mother.
What is one of the symptoms associated with microcephaly?
Impaired thinking and a small brain.
What are the steps involved in diagnosing microcephaly during pregnancy?
First trimester screening conducted between weeks 11-13 of pregnancy
If abnormal results or higher risk pregnancy, further diagnostic tests offered
Higher risk factors include being 35 years or older, previous birth defect pregnancy, or chronic diseases like lupus, diabetes, or epilepsy
Maternal blood screening measures hCG and pregnancy-associated plasma protein A (PAPP-A)
Ultrasound screening checks for extra fluid behind the baby's neck (nuchal translucency)
Extra fluid can indicate chromosomal disorders or heart defects
What is one possible cause of Guillain-Barre syndrome?
Possibly an autoimmune disorder.
What are the common symptoms of Guillain-Barre syndrome?
The common symptoms of Guillain-Barre syndrome include weakness and tingling in the extremities, which are often the first signs. In severe instances, paralysis may also occur.
What is the most common variant of GBS characterized by widespread demyelination of peripheral nerves?
Acute Inflammatory Demyelinating Polyradiculoneuropathy (AIDP)
What are the three variants of GBS?
The three variants of GBS are Acute Inflammatory Demyelinating Polyradiculoneuropathy (AIDP), Miller Fisher Syndrome (MFS), and Acute Motor Axonal Neuropathy (AMAN) and Acute Motor Sensory Axonal Neuropathy (AMSAN).
What are Acute Motor Axonal Neuropathy (AMAN) and Acute Motor Sensory Axonal Neuropathy (AMSAN)?
AMAN and AMSAN are variants of Guillain-Barre syndrome that involve damage to the axons (nerve fibers) instead of the myelin sheath. They are less common than the Acute Inflammatory Demyelinating Polyneuropathy (AIDP) variant.
What is Miller Fisher Syndrome (MFS)?
Miller Fisher Syndrome (MFS) is a rare variant of Guillain-Barré Syndrome (GBS). It is characterized by symptoms such as ataxia (lack of coordination), ophthalmoplegia (eye muscle weakness), and areflexia (loss of reflexes).
What is the purpose of CRISPR-Cas9 gene editing technology in preventing infection and microcephaly?
CRISPR-Cas9 gene editing technology aims to prevent infection and microcephaly by disrupting the binding of viruses to receptors.
What are the cardinal motor features of Parkinson's disease?
The cardinal motor features of Parkinson's disease are bradykinesia (slowness of movement), resting tremor, rigidity, and postural and gait impairment.
What are some non-motor features associated with Parkinson's disease (PD) diagnosis?
Neuropsychiatric features (such as depression and anxiety), dysautonomia, sleep disorders, sensory dysfunction, pain, and fatigue.
What are some pre-motor features of Parkinson's disease?
Impaired olfaction (can't smell), constipation, GI issues, depression, excessive daytime sleepiness, REM sleep behavior disorder (RBD), and changes in speech (recent findings, early changes).
What structures make up the basal ganglia system?
The basal ganglia system consists of the striatum (caudate and putamen), globus pallidus, subthalamic nucleus, substantia nigra, and pedunculopontine nucleus.
What are the two key pathological features of Parkinson's disease (PD)?
The two key pathological features of PD are the loss of dopaminergic neurons within the substantia nigra and the presence of protein aggregates called Lewy bodies.
What is the main cause of motor and non-motor symptoms in Parkinson's disease?
Loss of dopaminergic neurons within the substantia nigra pars compacta (SNc) is the main cause of motor and non-motor symptoms in Parkinson's disease.
What is the relationship between Lewy pathology and neuronal death in Parkinson's disease (PD)?
Lewy pathology alone cannot fully explain neuronal death in PD. Lewy bodies, which are aggregates of a-synuclein protein, can be found without neurodegeneration in individuals without PD. Additionally, neurodegeneration can occur in genetic forms of PD, such as PARKIN, without the presence of Lewy bodies.
What are some other pathological features observed in Parkinson's disease?
Neuronal loss can also occur in other brain regions, including the Locus coeruleus (noradrenaline), Nucleus basalis of Meynert (acetylcholine), Pedunculopontine nucleus (acetylcholine), and Raphe nucleus (serotonin).
What are some other pathological features observed in Parkinson's disease?
In addition to the characteristic alpha-synuclein aggregates, other types of protein aggregates can be observed in Parkinson's disease. These include amyloid plaques, similar to those found in Alzheimer's disease, and neurofibrillary tangles, also seen in Alzheimer's.
What are some pathological features associated with neuroinflammation?
Neuroinflammation is characterized by microglia activation and the presence of cytokines like TNF-alpha, IL-1beta, and IL-6 in the brain parenchyma.
What is the primary treatment for Parkinson's Disease?
Dopamine replacement therapy, specifically L-DOPA, is the primary treatment for Parkinson's Disease. It was first introduced in 1961 by Dr. Andre Barbeau. This therapy helps alleviate symptoms like bradykinesia (slowness of movement) and rigidity.
What is the mainstay of treatment for Parkinson's disease?
The mainstay of treatment for Parkinson's disease is L-dopa, often combined with a COMT inhibitor (Catechol-O-methyl transferase inhibitor), dopamine agonists, and MAO-B inhibitors.
What are the limitations of current PD treatment?
Motor symptoms inconsistently improve, such as tremor and postural instability.
Few non-motor symptoms improve, due to involvement of non-dopaminergic system
complications with disease progression
current therapies treat the systems but not slow nor stop the underlying neurodeg
Genetics and PD - General
1997 → Polymeropoulos and Nussbaum: identified 1st gene mutation (SNCA) associated with PD
10% of PD patients have family members who also have PD
Mutations in 7 genes have been identified as causes of PD
PD mutations with a mutation(s) in one of these genes are rare (only account for 3-5% of all PD
patients)
Genetic causes of PD - Autosomal dominant
SNCA → a-synuclein protein, responsible for protein aggregation (major component of Lewy bodies)
LRRK2 → leucine-rich repeat kinase 2, regulates neurite outgrowth, synaptic morphogenesis
VPS35 → vacuolar protein sorting 35, protein trafficking of endosomes
DNAJC13 → receptor-mediated endocytosis 8, protein trafficking of endosomes and transmembrane proteins
SNCA - PD
a-synuclein protein, responsible for protein aggregation (major component of Lewy bodies)
LRRK2 - PD
leucine-rich repeat kinase 2, regulates neurite outgrowth, synaptic morphogenesis
VPS35 - PD
vacuolar protein sorting 35, protein trafficking of endosomes
DNAJC13 - PD
receptor-mediated endocytosis 8, protein trafficking of endosomes and transmembrane
proteins
Genetic Causes - Autosomal recessive
PARK2 → parkin, works with PINK1 to dispose of damaged mitochondria
PINK1 → PTEN-induced putative kinase 1, works with PARK2
PARK7 → DJ1, redox sensor to protect from oxidative stress
ATP13A2 → ATPase type 13A2, works for protein degradation and involved in autophagy
PARK2 - PD
Parkin, works with PINK1 to dispose of damaged mitochondri
PINK1 - PD
PTEN-induced putative kinase 1, works with PARK2
PARK7 - PD
DJ1, redox sensor to protect from oxidative stress
ATP13A2 - PD
ATPase type 13A2, works for protein degradation and involved in autophagy
Physiological/normal pathways
Protein misfolds all the time, but it becomes a problem only if toxic oligomers are constantly made
Chaperone system helps unfolded polypeptide fold to non-toxic oligomers
If this keeps occurring, impaired proteostasis occurs and positive feedback occurs → toxic oligomers become amyloid fibrils, lead to saturated/overwhelmed pathways
A-synuclein then behaves like prions
Saturated/overwhelmed pathways
Toxic oligomers become amyloid fibrils, leading to saturated/overwhelmed pathways, impairing proteostasis
Oligomers disrupt proteostasis and UPR (unfolded protein response) occurs → misfolded proteins start to accumulate in the ER (causing ER stress) and cell dies through apoptosis
Particularly affects dopaminergic neurons within SN
Thought that toxic oligomers form pores that let calcium in (start to look like prion) and then the misfolded protein template replicates in cells
Influx of calcium disrupts cell functions
Toxic oligomers seeds itself in nearby cells after being released from affected cells, through vesicle release via endosomes or exocytosis
Once toxic oligomers enter a new cell via endocytosis, more misfolding is induced (cycle continues)
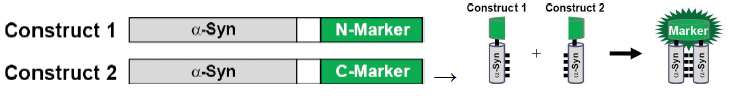
Protein fragment complementation
A-syn molecules are attached to either an N-marker or C-marker
See fluorescence if A-syn comes together and forms an oligomer (fluorescence non-existent on each half of GFP on its own)
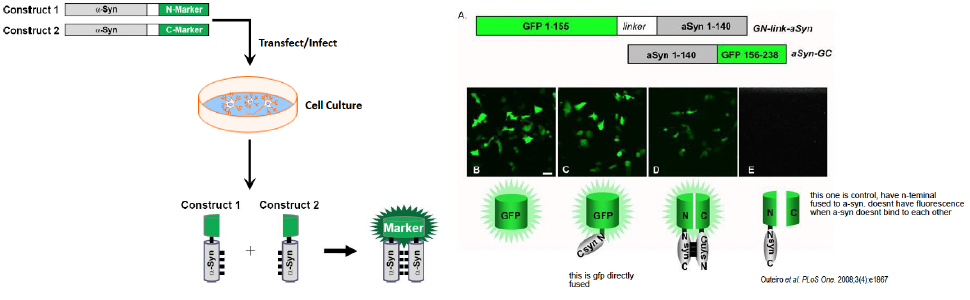
Cell culture model of a-syn oligomers
The cells with GFP alone or GFP attached to one a-syn molecule glowed green (controls)
Cells with two different GTP/a-syn constructs dimerized glowed green, but less than controls
Cell with a GTP/alpha-synuclein construct, when paired with a GTP "half" without alpha-synuclein, did not produce green fluorescence
C. elegans model of a-syn oligomers
A-syn oligomers can also be tested for in C. elegans (transparent, have characteristic sinusoidal movement (understanding of how asyn oligomerization affects movement)
Constructs are microinjected into the worm, and dimerization of the two GTP/a-syn constructs can be seen as a green glow
Rodent model of a-syn oligomers
Using AAV8, adeno-associated virus 8
Mouse model is injected (via virus vector) with the two alpha-synuclein constructs (one where alpha-syn is attached to the N-terminus of GFP and one where alpha-syn is attached to the C-terminus of GFP)
The virus injected infects the neurons and releases the DNA (with the alpha-syn constructs) in the neuron
Construct 1 and 2 come together → GFP fluoresces
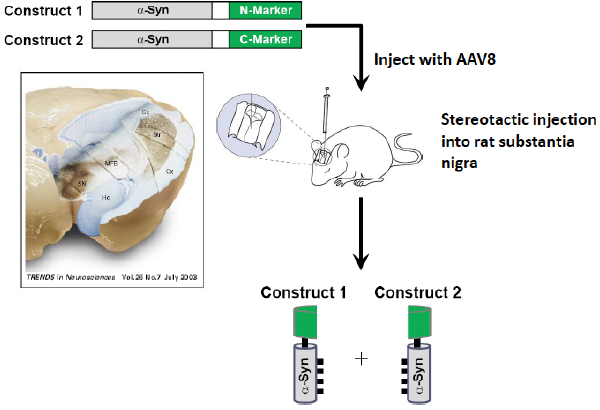
A-synuclein oligomers in the SN
V1S: not using GFP, but venus (GFP but different), and v1s is construct
If we inject construct 2 (SV2), we don’t see fluorescence
Second panel: when both constructs are injected, see fluorescence
When they put this construct into the rat brain, they’re able to target the substantia nigra and can see it inside neurons
A-syn mediated toxicity in the SN via immunostaining
Immunostaining lasts very long compared to fluorescence
V1S + SV2 (both constructs injected)
Injected side has fewer dopaminergic cells in the substantia nigra compared to the uninjected side → indicates that alpha-syn is forming oligomers
Similar amounts of DA loss for V1S SV2 and SV2 conditions as aSyn still oligomerizes
MPTP and its active metabolite - Toxicology model
MPP+ is toxic, inhibitor of complex I chain in mitochondria, leading to ER stress
Produces many of the clinical features of PD, which can be recovered by therapy (i.e. L-DOPA)
Selective loss of dopaminergic neurons in SNc
EM reveals lewy body-like inclusions, but not true lewy bodies
Rotenone - Toxicology model
Another mitochondrial complex I inhibitor in the mitochondria → leads to ER stress, loss of DA and other neurons
Infusion of rotenone via IV administration results in tremor, muscle weakness, hypokinesia
Also see loss of dopaminergic neurons as well as LB like inclusions
Mould and drosophila (1-octen-3-ol) - toxicology model
Epidemiology has suggested that mouldy buildings have many negative consequences (i.e. loss of balance and coordination)
Moulds consist of fungi that release volatile compounds, most prominently 1-octen-3-ol
Minimal dosages (0.5 ppm) can lead to Drosophila lethality after 12 days (50% mortality rate)
Prior to mortality, the same dose triggered movement deficits in flies 1 and 7 days after exposure
What is Elav-Gal4?
Gal4: transcriptional activator gene, produced in left parental line, whose expression is driven by Elav (promoter)
Elav: gene expressed specifically in post-mitotic neurons
Elav-Gal4: transgenic fly line
What is UAS-GFP?
Transgenic fly line that carries GFP under the control of the UAS promoter
Do not express GFP unless crossed with a fly line expressing the Gal4 protein
When crossed with a Gal4-expressing fly, GFP expression is induced in the tissue or cells where Gal4 is active
How do Elav-Gal4 and UAS-GFP work?
When crossed, shows fluorescence in gene of interest (in this case, dopaminergic neurons)
See that when exposed to 1-octen-3-ol for 24 hours, there’s decreases in dopaminergic neurons in various areas of the brain (decreased fluorescence) but increased DOPAC (metabolite of dopamine, see breakdown of dopamine)
See decreases in dopaminergic neurons via decrease of TH (TH is the gene that is expressed with GFP)
Also see deficits in motor abilities within the 24 hours
Some caveats of 1-octen-3-ol
1-octen-3-ol greatly reduced dopamine levels, but it’s used as a flavouring agent in prepared foods and perfumes
Theory is currently that lack of reuptake into vesicles may lead to more rapid breakdown of dopamine, leading to reactive oxygen species and damage
Just because this is the case for drosophila neurons, it may not be the case for human neurons
Overview of different points of toxicity
Oxidative stress in the mitochondria can cause a-syn aggregation, then toxic oligomers can start accumulating, and eventually the loss of these neurons
Genes affected:
LRRK2 and DJ-1 affects cells
Parkin and PINK1 affects the mitochondria
Environmental toxins like rotenone, iron and manganese may also cause oxidative stress
What is ER stress?
ER stress is when the ER cannot cope with the number of misfolded or unfolded proteins
Stress granules are formed as a way to lessen the burden of the ER (if present, indicative of ER stress)
PDI (protein disulfide isomerase) allows for normal protein folding
Dysfunction of this system indicative of ER stress → see positive feedback of stress within the ER
Tanzi’s neurodegeneration concept
Neurons contain APP, which can be cleaved to form amyloid beta bacteria biofilms. Biofilms are hard to eliminate and found in Alzheimer's patients. Borrelia bacteria (Lyme disease) is found in AD specimens, suggesting it as a potential origin. Alginate is a marker for bacterial biofilm in the brain.
Mechanism: Bacteria binds with TLR → activation of microglial cells with TLR2 → proinflammatory cytokines produced → neuroinflammation and potential damage to CNS occurs
Shifting perspectives - Gut microbiota and the brain
Brain inflammation might underlie PD and mood disorder
Gut microbiota interact with immune cells
Enteroendocrine cells found on gut lining produce neuroactive molecules that interact with the vagus nerve to ent
Dysbiosis
Definition: imbalance in the normal microbial community within your body
In adults, bacteria, viruses and eukaryotes population stay stable → changes with diet, disease, treatment and environmental effects
Gut viromes have >95% stability in their sequences
Dysbiosis and neurological diseases
Dysbiosis of human microbiome have been reported in subjects diagnosed with several neurological diseases
E.g. foecal and mucosa associated gut microbes are different between PD patients and healthy controls
Gut bacteria produce short-chain fatty acids (SCFAs) that can cause inflammation in the brain
The premise for the study onW mice and gut microbiota
Individuals with PD exhibit intestinal inflammation and GI abnormalities like constipation → often precede motor defects
Braak’s hypothesis → suggests that aberrant a-syn accumulation initiates in the gut and propagates via vagus nerve to brain like a prion
A-syn inclusions appear early in the enteric nervous system (ENS) and glossopharyngeal + vagal nerves, and vagotomized individuals are at reduced risk for PD
Thy1-aSyn (study of mice and gut microbiota)
Thy1-aSyn (alpha-synuclein-overexpressing, ASO) mouse displays progressive deficits in fine and gross motor function + gut motility defects
Defects in coordinated motor tasks particularly at 12 weeks of age
Thy1 is overexpressed in the presence of aSyn, only in neurons
What were the four tests to assess motor function?
beam traversal, pole descent, nasal adhesive removal and hindlimb clasping reflexes
What does SPF stand for?
specific pathogen free; these mice have normal gut bacteria and none of them cause
disease
What does GF stand for?
Germ-free, mice has no gut bacteria whatsoever
What does ASO stand for?
overexpression of a-syn
What was the staining for the study of mice and gut microbiota?
Neurotrace: stains all neurons blue
Phospho-Ser129-aSyn antibody: stains a-syn aggregates pink
Phospho-aSyn: stains phosphorylated a-syn green
Result from movement test - motor function in SPF mice
Normal wild type mice take about 5 seconds to cross a beam
Alpha synuclein mice take about 7 seconds to cross
Alpha synuclein mice have bradykinesia (slowness of movement)
Result from movement test - motor function in GF mice
Wild type GF mice take about 5 seconds to cross the beam
Alpha synuclein GF mice do not show significant slowness in crossing the beam
Swapping out gut bacteria eliminates slowness of movements
Result from movement test - coordinated movement
Wild type mice with intact gut bacteria come down a pole in about 6 seconds
Alpha synuclein mice take longer and exhibit bradykinesia
Wild type GF mice move faster than wild type controls
Alpha synuclein GF mice show similar movement to wild type mice
Result from movement test - tape removal test
Wild type mice with intact gut bacteria remove tape in about 2.5 seconds
Alpha synuclein mice take longer to remove tape
Eliminating gut bacteria reduces the time dramatically
Result from movement test - hindlimb clasping reflexes
Hindlimb clasping reflexes are slower with gut bacteria
Eliminating gut bacteria reduces the time
What did the staining reveal in the caudoputamen (part of the BG) and Substantia Nigra
The staining revealed that SPF-ASO mice had significantly more phosphorylated aSyn and aSyn aggregates compared to GF-ASO mice. This suggests that the increase in insoluble aggregates of aSyn was influenced by the gut microbiota. Triton X-100, a detergent, was used to dissolve proteins. Triton soluble monomeric aSyn remained in solution, while triton-insoluble phosphorylated aSyn remained in a pellet.
A-syn dependent microglia activation
Iba-1 is a stain specific for microglia cells.
Microglia morphology changes with activation (bigger cell bodies, shorter processes)
Before activation: thin cell bodies with lots of branches
After activation: round amoeboid cells with less branches
Proinflammatory cytokines are secreted by activated microglia cells → TNF-a and IL-6
Tissues from the CP of SPF-ASO mice had more pro inflammatory cytokines compared to GF-ASO mice.
High levels of TNF-a and IL-6 are often found in the brains of PD patients
CD11b+ cells are antigen markers for microglia cells, and gene analysis of their RNA also showed increased inflammatory cytokine levels
Abx administration to SPF mice
Antibiotic cocktail that kills microbiota after birth
Improved motor function compared to untreated mice
Reduced aSyn-dependent motor dysfunction
Introduction of bacteria to GF mice decreased motor function
Does microglia activation require short chain fatty acids (SCFAs)?
Yes, microglia activation can be triggered by SCFAs. SCFAs, which are produced by gut bacteria, have been found to potentially activate microglia during a viral infection. Examples of SCFAs include acetate, propionate, and butyrate – when these 3 were introduced to GF-ASO and GF-WT animals, they began having movement disorders and microglia activation
What is the faecal analysis study?
The faecal analysis study is a research study conducted to investigate the altered microbiomes observed in Parkinson's disease (PD) patients. The study involved collecting faecal samples from 6 PD patients and 6 healthy individuals (control group). These samples were then transplanted into separate groups of germ-free (GF) recipient animals through oral gavage.
What is the method for the faecal analysis study?
Using oral gavage (dosing) method to transplant fecal microbiota from both PD patients and control into mice. Raw data was collected with bacterial DNA extraction. 16s rRNA sequencing was used to conduct analysis. Data was presented using OTUs
What were the results from the faecal analysis study?
The results from the faecal analysis study showed that mice with PD patient's microbiota exhibited clustered OTUs, indicating that PD microbe groups were very similar to each other in terms of microbial content and diversity. Additionally, PD donor-derived microbiota displayed significantly altered SCFA profiles, with lower acetate concentration and higher propionate and butyrate levels. It was also found that alpha-synuclein overexpression caused distinct alterations to the gut microbiome after transplantation. Furthermore, the study revealed that PD microbiota only induced disease symptoms in genetically susceptible hosts (ASO animals). Lastly, gut microbiomes from PD patients demonstrated greater motor deficits in mouse models, providing evidence for the contribution of microbiota to synucleinopathies.