CHEM 120 Final Review
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
24 Terms
How to find mass given density?
mass = density x volume
Fahrenheit to Celcius conversion equation
C=(F-32)/1.8
Which of the following are metric units of volume?
i. mL
ii. L
iii. cm^3
iv. m
v. kg
i., ii., and iii.
When paper is burned, the mass of the remaining ash is less than the mass of the original paper. Which of the following is the best explanation for the apparent violation of the Law of Conservation of Mass?
When invisible substances are taken into account, the total mass of the reactants is equal to that of the total mass of the products.
Which of the following is/are heterogeneous substances?
i. gasoline
ii. gold bar
iii. a freshly opened can of cola
iv. potato chips
v. a bacon strip
iii., iv., and v.
A 60kg astronaut travels from the earth to the moon. If the gravitational attraction on the moon is 1/6 of that on earth, what will be the mass of the astronaut on the moon?
60kg
Under what condition is the Law of Conservation of Energy not obeyed?
Nuclear changes
Convert 1.57g to kg, cg, and mg
0.00157kg, 157cg, 1570mg
(T/F): As a positively charged object moves toward a negatively charged object, its potential energy increases.
F
When room temperature solutions of vinegar and drain cleaner are combined, the temperature of the resulting solution is greater than room temperature. What is the best explanation for this apparent violation of the Law of Conservation of Energy?
The total energy of the original solutions is equal to the total energy of the resulting solution plus the heat energy.
(T/F): Electrostatic forces are responsible for the energy absorbed or released in chemical charges.
T
(T/F): The region in space where magnetic or electrostatic forces are effective is called a force field.
T
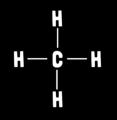
What is methane’s molecular geometry?
Tetrahedral
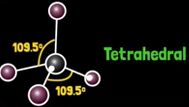
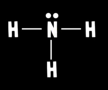
What is ammonia’s molecular geometry?
Trigonal pyramidal
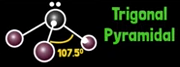
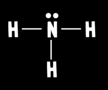
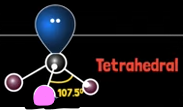
What is ammonia’s electron geometry?
Tetrahedral
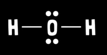
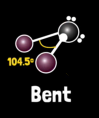
What is water’s molecular geometry?
Bent
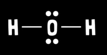
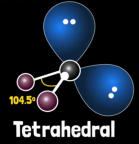
What is water’s electron geometry?
Tetrahedral
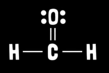
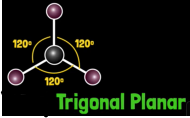
What is formaldehyde molecular geometry?
Trigonal planar
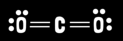
What is carbon dioxide’s molecular geometry?
Linear
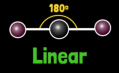
How many bonds and lone pairs are in a TETRAHEDRAL?
4 Bonds
0 Lone Pairs
How many bonds and lone pairs are in a TRIGONAL PYRAMIDAL structure?
3 Bonds
1 Lone Pair
How many bonds and lone pairs are in a BENT structure?
2 Bonds
2 Lone Pairs
How many bonds and lone pairs are in a TRIGONAL PLANAR structure?
3 Bonds
0 Lone Pairs
How many bonds and lone pairs are in a LINEAR structure?
2 Bonds
0 Lone Pairs