6. variable oxidation state
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
transition metals exist in variable oxidation states
switching between oxidation states is a redox reaction where the metal ions are either oxidised or reduced

vanadium oxidation states
+2 +3 +4 +5
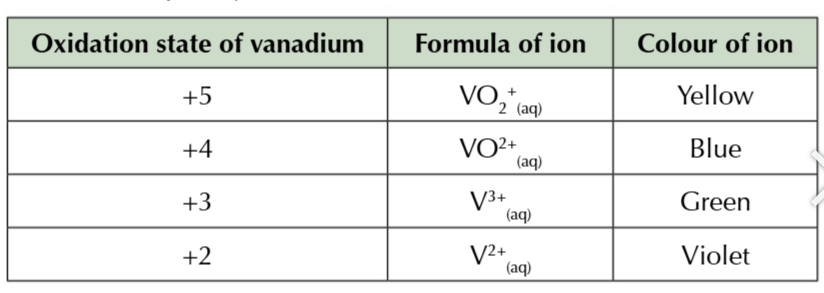
vanadium V can be reduced by adding it to zinc metal in an acidic solution
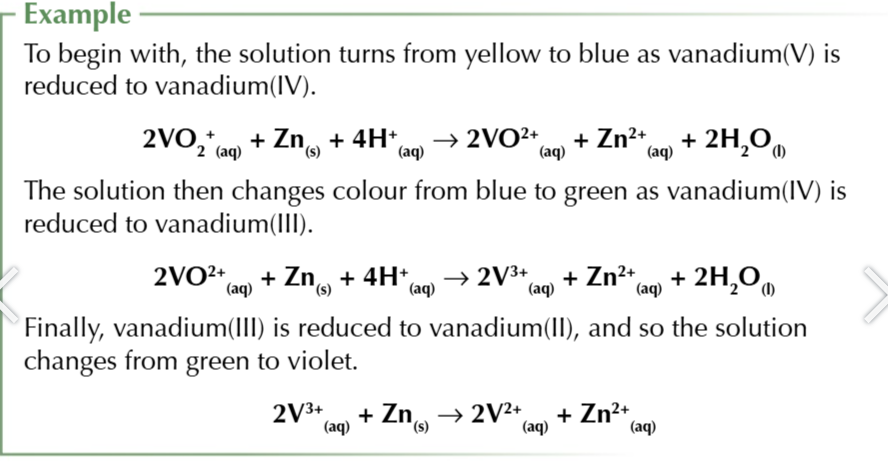
redox potential
how easily an ion or atom is reduced to a lower oxidation state
redox potential is the same as
electrode potentials
the more positive the redox potential
the less stable the ion will be and so the more likely it is to be reduced
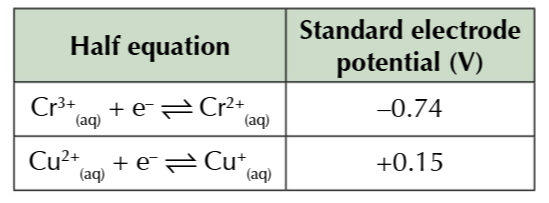
Copper II has a redox potential of +0.15V, so its less stable than Chromium and more likely to be reduced than chromium
redox potentials in a table
are standard electrode potentials measured with reactants at concentration of 1 mol-3 against SHE under standard conditions
redox potential of an ion wont always be the same as its standard electrode potential
it can vary depending on the environment of the ion
standard electrode potentials are measured in aqueous solutions
so any ions will be surrounded by water ligands
different ligands may make the redox potential larger or smaller
depending on how many will bind to the metal ion in a particular oxidation state
some ions need H+ ions present in order to be reduced
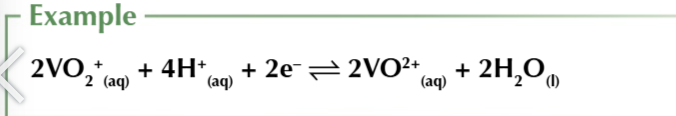
other ions release OH- ions into solution when they’re reduced
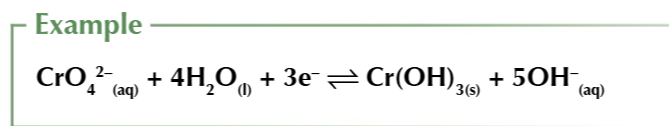
silver is a transition metal commonly found in +1 oxidation state Ag+
it is easily reduced to silver metal

tollens reagent preparation:
adding just enough ammonia solution to silver nitrate to form a colourless solution containing [Ag(NH3)2]+
when tollens is added to aldehydes
it reacts to give a silver mirror inside the test tube
the aldehyde is oxidised to a carboxylate anion
Ag+ ions are reduced to silver metal
RCHO + [Ag(NH3)2]+ + 3OH- →
RCOO- +2Ag +4NH3 + 2H2O
tollens reagent cant oxidise ketones
so it will not react to form a silver mirror